Exhibit 99.1
Media Release

IMV Inc. Announces Second Quarter 2019 Financial Results and Provides Company Update
-
Presented updated data from Phase 2 monotherapy arm of DeCidE1 study at ASCO 2019; DPX-Survivac exhibited durable clinical benefit with progression-free survival in patients with advanced recurrent ovarian cancer
-
Reported positive new data from Phase 2 SPiReL combination study of DPX-Survivac in r/r DLBCL, including long-lasting complete responses
-
The 16 additional patients in the expanded monotherapy arm of the Phase 1b/2 DeCidE1 Clinical Study in Advanced Recurrent Ovarian Cancer are enrolled
-
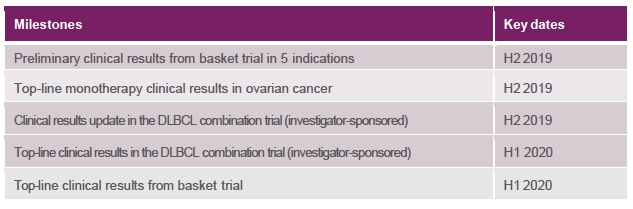
Additional data expected from three Phase 2 studies of DPX-Survivac by year-end, including as monotherapy in ovarian cancer and as combination therapy in r/r DLBCL and multiple other solid tumor types
-
Management to host conference call and webcast tomorrow at 8:00 am ET
Dartmouth, Nova Scotia; August 8, 2019 - IMV Inc. (TSX: IMV; TSX: NASDAQ), a clinical stage immunotherapy company, today released its financial and operational results for the second quarter ended June 30, 2019.
“At IMV, we are leveraging DPX, our no-release delivery technology that enables us to program immune cells in vivo. We continue to believe this mechanism offers potential to produce a new class of immunotherapies that elicit a more rapid, robust and sustained immune response,” said Frederic Ors, IMV's Chief Executive Officer. “In the second quarter, we reported important updates from two Phase 2 studies of our lead program, DPX-Survivac, our T cell-activating immunotherapy harnessing the power of this approach to target survivin, which is present in more than 20 solid and hematological tumor types. These results continue to validate our DPX platform and this novel target, with encouraging signs of anti-tumor activity contributing to the body of data we have accumulated to demonstrate DPX-Survivac’s potential in hard-to-treat cancers, both as a monotherapy and in combination with other agents. We look forward to providing additional data from this program by year-end, including top-line results from our Phase 2 study evaluating DPX-Survivac as a monotherapy in ovarian cancer and additional results from our Phase 2 r/r DLBCL and basket trial, evaluating DPX-Survivac in combination with Keytruda in multiple solid tumor indications.”
DPX-Survivac Clinical Program Updates:
Phase 1b/2 DeCidE1 Clinical Study in Advanced Recurrent Ovarian Cancer
In June 2019, IMV presented new data at the 2019 American Society of Clinical Oncology (ASCO) annual meeting from DeCidE1, its ongoing Phase 1b/2 study evaluating the safety and efficacy of DPX-Survivac and intermittent low-dose cyclophosphamide (CPA), with and without epacadostat, in advanced recurrent ovarian cancer. These results expand on data previously reported from this study, which exhibited a durable response. Highlights of the new data from evaluable patients in the Phase 2 monotherapy arm of the trial include:
-
Five out of seven patients showed signs of treatment benefits, including reduction of target lesions in two patients;
-
Three out of four patients with low tumor burden showed stable disease, including two with tumour regression at first CT scan;
-
Of the five patients evaluable for T cell responses, all showed survivin-specific T cell activation in the blood; and
-
Treatments were well-tolerated supporting our views of the favourable safety profile of our approach.
Additionally, longer-term follow-up data from the Phase 1b portion of the study continued to demonstrate the prolonged duration of clinical benefits, surpassing two years of progression-free survival from previous treatments, including platinum-based chemotherapy.
We have enrolled 16 additional patients in the expanded monotherapy arm of the DeCidE1 trial. IMV expects to provide the top-line clinical results from this study before the end of 2019.
Phase 2 SPiReL Study of DPX-Survivac in Combination with KEYTRUDA® in Relapsed/Refractory Diffuse Large B-Cell Lymphoma (DLBCL)
In June 2019, IMV reported updated data from SpiRel, its ongoing investigator-sponsored Phase 2 clinical trial assessing DPX-Survivac in combination with intermittent low dose cyclophosphamide and Merck’s checkpoint inhibitor Keytruda, linking antitumor activity with T cell responses that correlate with expression of survivin.
At the first "on treatment" interim assessment, five of the first six patients demonstrated clinical benefit, including four patients with tumor regressions. Two patients reached a complete radiological response, one exhibited a partial response and two reached stable disease while on the study. In addition, the study continued to demonstrate an acceptable safety profile for the two therapies in combination.
Based on these data, IMV agreed with the principal investigator to increase the number of sites recruiting patients from 5 to 9. As of August 8, 2019, investigators had enrolled 12 patients across four different clinical sites in Canada. Additional patients are being screened and IMV expects to give another update on this trial before the end of 2019 and report top-line clinical data from this study in the first half of 2020.
Phase 2 Basket Trial of DPX-Survivac in Combination with KEYTRUDA® in Multiple Solid Tumor Indications
Fifteen patients have been enrolled to date, while screening and enrollment of patients is ongoing across nine clinical sites in the U.S. and Canada for five cohorts of patients with bladder, liver (hepatocellular carcinoma), ovarian, or non-small cell lung (NSCLC) cancers, as well as tumors shown to be positive for the microsatellite instability high (MSI-H) biomarker.
Patients have been treated in every cohort and IMV expects to report preliminary clinical results on several of the solid tumor indications included in this basket trial before the end of 2019.
Operational Highlights:
Modification of $5M loan agreement with the province of Nova Scotia. Previously, the entire loan was payable on August 9, 2020. Per this modification, the Corporation will now repay the loan over 60 months starting in January 2021. All the other terms remain the same. This revised repayment schedule will allow IMV to focus its cash resources towards developing its clinical programs.
Upcoming Milestones:
Overview of Q2 2019 Financial Results
(In Canadian dollars)
At June 30, 2019, the Corporation had cash and cash equivalents of $26.9M and working capital of $28.3M, compared with $14.9M and $12.2M, respectively at December 31, 2018. Management believes that the Corporation’s cash resources of $26.9M and its additional potential cash resources of $3.1M will be sufficient to fund operations up to Q4-2020. For the six-month period ended June 30, 2019, IMV's cash burn rate (defined as net loss and comprehensive loss adjusted for charges to operations not involving cash as described in the statement of cash flows) was $10.4 million. Based on the current business plan, the Corporation forecasts the quarterly cash burn rate to be between $5 million and $6 million for the remaining of 2019.
The net loss and comprehensive loss of $5.0M ($0.10 per share) for the three-month period ended June 30, 2019, was $0.2M lower than the net loss and comprehensive loss for three-month period ended June 30, 2018. This relates mainly to a $0.4M decrease in general and administrative expenses and a $0.9M increase in government assistance partly compensated by a $1.2M
increase in research and development (R&D) expenses, in the three-month period ended June 30, 2019.
For the six-month period ended June 30, 2019, the net loss and comprehensive loss of $11.0M was $2.7M higher than the net loss and comprehensive loss for six-month period ended June 30, 2018. This relates mainly to a $3.3M increase in R&D expenses and a $0.2M increase in general and administrative expenses partly compensated by $1.0M increase in government assistance in the six-month period ended June 30, 2019. As of August 8, 2019, the number of issued and outstanding common shares was 50,612,125 and a total of 1,997,232 stock options, warrants, and deferred share units were outstanding at that date.
The Corporation's unaudited interim condensed consolidated results of operations, financial condition and cash flows for the three and six-months ended June 30, 2019 and the related management's discussion and analysis (MD&A) are available on SEDAR at www.sedar.com and on EDGAR at www.sec.gov/edgar.
Conference Call and Webcast Information
IMV will host a conference call and webcast tomorrow at 8:00 am ET to discuss these results and provide an update on the company.
Financial analysts are invited to join the conference call by dialing (844) 461-9932 (U.S. and Canada) or (636) 812-6632 (international) using the conference ID: 7148568.
Other interested parties will be able to access the live audio webcast at this link: https://ir.imv-inc.com/events-and-presentations. The webcast will be recorded and available on the IMV website for 30 days following the call.
About IMV
IMV Inc. is a clinical stage biopharmaceutical company dedicated to making immunotherapy more effective, more broadly applicable, and more widely available to people facing cancer and other serious diseases. IMV is pioneering a new class of immunotherapies based on the Corporation’s proprietary drug delivery platform. This patented technology leverages a novel mechanism of action that enables the programming of immune cells in vivo, which are aimed at generating powerful new synthetic therapeutic capabilities. IMV’s lead candidate, DPX-Survivac, is a T cell-activating immunotherapy that combines the utility of the platform with a target: survivin. IMV is currently assessing DPX-Survivac as a monotherapy in advanced ovarian cancer, as well as a combination therapy in multiple clinical studies with Merck. Connect at www.imv-inc.com
IMV Forward-Looking Statements
This press release contains forward-looking information under applicable securities law. All information that addresses activities or developments that we expect to occur in the future is forward-looking information. Forward-looking statements are based on the estimates and opinions of management on the date the statements are made. However, they should not be regarded as a representation that any of the plans will be achieved. Actual results may differ materially from those set forth in this press release due to risks affecting the Corporation, including access to capital, the successful completion of clinical trials and receipt of all regulatory approvals. IMV Inc. assumes no responsibility to update forward-looking statements in this press release except as required by law. These forward-looking statements involve known and unknown
risks and uncertainties and those risks and uncertainties include, but are not limited to, our ability to access capital, the successful and timely completion of clinical trials, the receipt of all regulatory approvals and other risks detailed from time to time in our ongoing quarterly filings and annual information form. Investors are cautioned not to rely on these forward-looking statements and are encouraged to read IMV’s continuous disclosure documents, including its current annual information form, as well as its audited annual consolidated financial statements which are available on SEDAR at www.sedar.com and on EDGAR at www.sec.gov/edgar.
###
Contacts for IMV:
INVESTOR RELATIONS:
Marc Jasmin, Senior Director, Investor Relations and Communications
O: (902) 492-1819 ext : 1042
M: (514) 617-9481 E: [email protected]
Josh Rappaport, Director, Stern IR
O: (212) 698-8678
E: [email protected]
|
| IMV INC. |
| Unaudited Interim Condensed Consolidated Statements of Loss and Comprehensive Loss |
| (In thousands of Canadian dollars, except shares and per share amounts) |
|
|
|
|
|
|
|
|
|
|
|
|
| |
Three-month ended |
|
|
|
|
|
Six-month ended |
|
| |
|
|
|
June 30 |
|
|
|
|
|
June 30 |
|
| |
2019 |
|
|
2018 |
|
|
2019 |
|
|
2018 |
|
| |
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
| Revenue |
|
|
|
|
|
|
|
|
|
|
|
| Subcontract revenue |
6 |
|
|
17 |
|
|
14 |
|
|
45 |
|
| Interest Income |
180 |
|
|
112 |
|
|
254 |
|
|
181 |
|
| Total revenue |
186 |
|
|
129 |
|
|
268 |
|
|
226 |
|
| Expenses |
|
|
|
|
|
|
|
|
|
|
|
| Research and development |
3,803 |
|
|
2,605 |
|
|
7,816 |
|
|
4,487 |
|
| General and administrative |
2,184 |
|
|
2,640 |
|
|
4,144 |
|
|
3,930 |
|
| Government assistance |
(1,142 |
) |
|
(189 |
) |
|
(1,488 |
) |
|
(464 |
) |
| Accreted interest |
392 |
|
|
269 |
|
|
790 |
|
|
536 |
|
| Total operating expenses |
5,232 |
|
|
5,325 |
|
|
11,262 |
|
|
8,489 |
|
| Net loss and comprehensive loss |
(5,051 |
) |
|
(5,196 |
) |
|
(10,994 |
) |
|
(8,263 |
) |
| Basic and diluted loss per share |
(0.10 |
) |
|
(0.12 |
) |
|
(0.23 |
) |
|
(0.19 |
) |
| Weighted-average shares outstanding |
50,601,866 |
|
|
43,001,620 |
|
|
48,667,904 |
|
|
42,539,304 |
|
|
| IMV INC. |
| Unaudited Interim Condensed Consolidated Statements of Financial Position |
| (In thousands of Canadian dollars, except shares and per share amounts) |
|
|
|
|
|
|
|
|
| |
|
June 30, |
|
|
|
December 31, |
|
| |
|
2019 |
|
|
|
2018 |
|
| Assets |
|
|
|
|
|
|
|
| Current assets |
|
|
|
|
|
|
|
| Cash and cash equivalents |
$ |
26,904 |
|
|
$ |
14,895 |
|
| Accounts receivable |
|
1,285 |
|
|
|
1,337 |
|
| Prepaid expenses |
|
4,343 |
|
|
|
2,699 |
|
| Investment tax credits receivable |
|
1,750 |
|
|
|
1,111 |
|
| Total current assets |
|
34,282 |
|
|
|
20,042 |
|
| Property and equipment |
|
3,063 |
|
|
|
2,883 |
|
| Total assets |
$ |
37,345 |
|
|
$ |
22,925 |
|
| |
|
|
|
|
|
|
|
| Liabilities and Equity |
|
|
|
|
|
|
|
| Current liabilities |
|
|
|
|
|
|
|
| Accounts payable and accrued liabilities |
$ |
5,733 |
|
|
$ |
7,575 |
|
| Amounts due to directors |
|
63 |
|
|
|
49 |
|
| Current portion of long-term debt |
|
86 |
|
|
|
81 |
|
| Current portion of lease obligations |
|
95 |
|
|
|
90 |
|
| Total current liabilities |
|
5,977 |
|
|
|
7,795 |
|
| Lease obligation |
|
1,259 |
|
|
|
1,308 |
|
| Deferred share units |
|
1,046 |
|
|
|
1,436 |
|
| Long-term debt |
|
7,972 |
|
|
|
8,069 |
|
| Total liabilities |
|
16,254 |
|
|
|
18,608 |
|
| Equity |
|
21,091 |
|
|
|
4,317 |
|
| Total liabilities and equity |
$ |
37,345 |
|
|
$ |
22,925 |
|

