Exhibit 99.2

Management’s Report on Financial Position and Operating Results
For the three and nine-months ended September 30, 2018
Exhibit 99.2

Management’s Report on Financial Position and Operating Results
For the three and nine-months ended September 30, 2018
LETTER TO SHAREHOLDERS
Dear Fellow Shareholders,
During the third quarter of 2018, we continued to demonstrate DPX-Survivac’s ability to generate novel targeted anti-cancer T cell responses. We believe that this trend should be a key value driver for IMV and a potential cornerstone for future immunotherapy combinations. From the initial positive data in our lymphoma trial to our collaboration with Merck on a phase 2 trial in multiple indications, our clinical program is well positioned to expand the range of patients who may benefit from novel immunotherapies, particularly in underserved cancers.
We anticipate continued progress on several important milestones over the next year, which include:
Topline data from the higher dosing cohort in our clinical trial with Incyte;
Topline data from our triple combination phase 2 trial with Merck in diffuse large B-cell lymphoma (DLBCL);
Initial data from our second triple combination phase 2 trial with Merck in ovarian cancer; and
Preliminary data from our phase 2 basket trial.
DPX-Survivac clinical program update
Ovarian Cancer
Our DECIDE1 (DPX-Survivac with low dose cyclophosphamide and Epacadostat) phase 1b/2 clinical trial with Incyte reached two significant milestones: completion of enrollment of both phase 1b dosing cohorts; and treatment of the first patient in the phase 2 cohort. We expect to announce topline data from the Phase 1b portion of the trial in Q4 2018.
Diffuse large B-cell lymphoma (DLBCL)
IMV announced the first clinical data from the combination of DPX-Survivac and mCPA with a checkpoint inhibitor. The initial data came from the investigator-sponsored phase 2 trial evaluating DPX-Survivac, low dose cyclophosphamide, and Merck's Keytruda® (pembrolizumab) in patients with persistent or recurrent/refractory DLBCL. Significant anti-cancer activity was seen in three of the first four evaluable patients, along with a tolerable safety profile.
Merck Collaboration: Phase 2 Basket Trial
IMV announced a collaboration with Merck in a phase 2 trial that will evaluate the safety and efficacy of DPX-Survivac in combination with low-dose cyclophosphamide and Merck's Keytruda in patients with select advanced or recurrent solid tumors across five different indications (lung (NSCLC), bladder, liver (HCC), MSI-H, ovarian). In the fourth quarter of 2018, investigators plan to initiate enrollment of more than 200 patients at multiple centers across the U.S. and Canada.
Operational highlights of Q3 2018 to-date include:
Opening of new facility in Dartmouth, Nova Scotia: The new premises features upgraded facilities and equipment as well as increased laboratory size and capacity. We have now nearly tripled our functional work space to allow for expanding business activities in the coming years.
Cash position: As of September 30, 2018, cash and cash equivalents and short-term investments were $20 million compared to $15 million as of December 31, 2017.
We are still making great progress and are grateful for the continued support of our partners, Incyte and Merck, as well as our shareholders and employees. We look forward to another productive quarter.

Frederic Ors
Chief Executive Officer
MANAGEMENT DISCUSSION AND ANALYSIS (“MD&A”)
The following analysis provides a review of the unaudited interim condensed consolidated results of operations, financial condition and cash flows for the three and nine-month period ended September 30, 2018 (“Q3 2018”), with information compared to the three and nine-month period ended September 30, 2017 (“Q3 2017”), for IMV Inc. - formerly Immunovaccine Inc. (“IMV” or the “Corporation”). This analysis should also be read in conjunction with the information contained in the audited annual consolidated financial statements and related notes for the years ended December 31, 2017 and December 31, 2016.
The Corporation prepares its unaudited interim condensed consolidated financial statements in accordance with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board (IASB). Management is responsible for the preparation of the consolidated financial statements and other financial information relating to the Corporation included in this report. The Board of Directors is responsible for ensuring that management fulfills its responsibilities for financial reporting. In furtherance of the foregoing, the Board of Directors has appointed an Audit Committee comprised of independent directors. The Audit Committee meets with management and the auditors in order to discuss results of operations and the financial condition of the Corporation prior to making recommendations and submitting the consolidated financial statements to the Board of Directors for its consideration and approval for issuance to shareholders. The information included in this MD&A is as at November 1, 2018, the date when the Board of Directors approved the Corporation’s unaudited interim condensed consolidated financial statements for the three and nine-month period ended September 30, 2018, on the recommendation of the Audit Committee.
Amounts presented in this MD&A are approximate and have been rounded to the nearest thousand except for per share data. Unless specified otherwise, all amounts are presented in Canadian dollars.
Additional information regarding the business of the Corporation, including the Annual Information Form of the Corporation for the year ended December 31, 2017 (the “AIF”) and included in the Corporation’s registration statement on Form 40-F filed with the U.S. Securities and Exchange Commission, is available on SEDAR at www.sedar.com and on EDGAR at www.sec.gov/edgar.
FORWARD-LOOKING STATEMENTS
Certain statements in this MD&A may constitute “forward-looking” statements which involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements of the Corporation, or industry results, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. When used in this MD&A, such statements use such words as “will”, “may”, “could”, “intends”, “potential”, “plans”, “believes”, “expects”, “projects”, “estimates”, “anticipates”, “continue”, “potential”, “predicts” or “should” and other similar terminology. These statements reflect current expectations of management regarding future events and operating performance and speak only as of the date of this MD&A. Forward looking statements include, among others:
the Corporation’s business strategy;
statements with respect to the sufficiency of the Corporation’s financial resources to support its activities;
potential sources of funding;
the Corporation’s ability to obtain necessary funding on favorable terms or at all;
the Corporation’s expected expenditures and accumulated deficit level;
the Corporation’s expected outcomes from its ongoing and future research and research collaborations;
the Corporation’s exploration of opportunities to maximize shareholder value as part of the ordinary course of its business through collaborations, strategic partnerships and other transactions with third parties,
the Corporation’s plans for the research and development of certain product candidates;
the Corporation’s strategy for protecting its intellectual property;
the Corporation’s ability to identify licensable products or research suitable for licensing and commercialization;
the Corporation’s ability to obtain licences on commercially reasonable terms;
the Corporation’s plans for generating revenue;
the Corporation’s plans for future clinical trials; and
the Corporation’s hiring and retention of skilled staff.
Forward-looking statements involve significant risks and uncertainties, should not be read as guarantees of future performance or results, and will not necessarily be accurate indications of whether or not such results will be achieved. A number of factors could cause actual results to differ materially from the results discussed in the forward-looking statements, including, but not limited to, the factors discussed in the AIF, under the heading “Risk Factors and Uncertainties”. Although the forward-looking statements contained in this MD&A are based upon what management of the Corporation believes are reasonable assumptions, the Corporation
3
cannot provide any assurance to investors that actual results will be consistent with these forward-looking statements and should not be unduly relied upon by investors.
Actual results and developments are likely to differ, and may differ materially, from those expressed or implied by the forward-looking statements contained in this MD&A. Such statements are based on a number of assumptions which may prove to be incorrect, including, but not limited to, assumptions about:
obtaining additional funding on reasonable terms when necessary;
positive results of pre-clinical studies and clinical trials;
the Corporation’s ability to successfully develop existing and new products;
the Corporation’s ability to hire and retain skilled staff;
the products and technology offered by the Corporation’s competitors;
general business and economic conditions;
the Corporation’s ability to protect its intellectual property;
the Corporation’s ability to manufacture its products and to meet demand; and
regulatory approvals.
These statements reflect management’s current beliefs and are based on information currently available to management. The information contained herein is dated as of November 1, 2018, the date of the Board’s approval of the Q3 2018 unaudited interim condensed consolidated financial statements and of the MD&A. For additional information on risks, uncertainties and assumptions, including a more detailed assessment of the risks that could cause actual results to materially differ from current expectations, please refer to the AIF of IMV filed on SEDAR at www.sedar.com and included in the registration statement on Form 40-F filed on EDGAR at www.sec.gov/edgar.
CORPORATE OVERVIEW
IMV is a clinical stage biopharmaceutical company dedicated to making immunotherapy more effective, more broadly applicable, and more widely available to people facing cancer and other serious diseases. IMV is pioneering a new class of immunotherapies based on the Corporation’s proprietary drug delivery platform. This patented technology leverages a novel mechanism of action that enables the programming of immune cells in vivo, which are aimed at generating powerful new synthetic therapeutic capabilities.
The Corporation’s first cancer immunotherapy uses survivin-based peptides licensed from Merck KGaA, on a world-wide exclusive basis, formulated in DPX. Survivin is a well characterized and recognized tumor associated antigen known to be expressed during fetal development and across most tumour cell types, but is rarely present in normal, non-malignant adult cells. It has been shown that survivin was expressed in all 60 different human tumour lines used in the National Cancer Institute’s cancer drug-screening program.
DPX-Survivac is currently being tested in:
a co-funded phase 1b/2 clinical trial with Incyte Corporation (“Incyte”), which evaluates the combination of DPX- Survivac with Incyte’s investigational oral indoleamine 2,3-dioxygenase 1 (“IDO1”) inhibitor, epacadostat, in ovarian cancer patients;
two investigator-sponsored phase 2 clinical trials in combination with checkpoint inhibitor Keytruda® (pembrolizumab) of Merck & Co Inc. (“Merck”) in patients with recurrent, platinum-resistant and sensitive ovarian cancer and in patients with measurable or recurrent diffuse large B cell lymphoma (“DLBCL”); and
a phase 2 basket trial in combination with Merck’s Keytruda® (pembrolizumab), in patients with select advanced or recurrent solid tumours in bladder, liver (hepatocellular carcinoma), ovarian or non-small-cell lung (NSCLC) cancers, as well as tumours shown to be positive for the microsatellite instability high (MSI-H) biomarker.
In infectious disease vaccine applications, the Corporation has completed a demonstration phase 1 clinical trial with a target against the respiratory syncytial virus (“RSV”). The Corporation also has a commercial licencing agreement with Zoetis for the development of two cattle vaccines and is also conducting several research and clinical collaborations, including a collaboration with the Dana-Farber Cancer Institute (“Dana-Farber”) for Human Papillomavirus (“HPV”) related cancers and with Leidos, Inc. (“Leidos”) in the United States for the development of vaccine candidates for malaria and the Zika virus.
4
The common shares of the Corporation are listed on the Nasdaq Stock Market LLC and on the Toronto Stock Exchange under the symbol “IMV”.
BUSINESS MODEL AND STRATEGY
IMV is dedicated to making immunotherapy more effective, more broadly applicable and more widely available to people facing cancer. The Corporation’s lead product, DPX-Survivac, has demonstrated the ability to induce prolonged T cell activation leading to tumor regressions in advanced ovarian cancer and is currently being used in clinical trials in combination with checkpoint inhibitors from the Corporation’s collaborators, Incyte and Merck. The target of this T cell therapy is broadly applicable to many different cancers. The novel mechanism of action of the underlying delivery platform, DPX, is to promote uptake and extend exposure of antigens to cells of the immune system, which enhances and sustains immune responses. This allows IMV to leverage this technology to develop next-generation immunotherapy treatments and become a preferred partner in combination trials in hard to treat cancers.
IMV believes that the principles behind a successful cancer immunotherapy should include a targeted antigen and an effective formulation and delivery technology, combined with a complementary therapeutic strategy. Antigens used in DPX-Survivac are believed to specifically target tumor cells without harming normal, healthy cells. These antigens are combined with the Corporation’s DPX platform in an effort to optimize the delivery of these antigens to the immune system, resulting in an enhanced immune activation. To be successful against cancer, the Corporation believes antigens must be administered in the right therapeutic setting, which includes a combination of therapies that help target various aspects of cancer. IMV believes that the effect of the therapy may be enhanced if an immune modulator is used simultaneously to prevent a patient’s immune system from overriding the positive response to the antigen. The Corporation’s goal in immuno-oncology is to advance its proprietary therapies in combination trials with pharmaceutical and large biotechnology companies to establish strategic partnerships and support further development and commercialization.
In collaboration with commercial and academic partners, the Corporation is also expanding the application of DPX as a delivery platform for other applications. Pre-clinical and clinical studies have indicated that the platform may allow for the development of enhanced vaccines for a wide range of infectious diseases by generating a stronger and more durable immune response more quickly than is possible with existing delivery methods.
The Corporation intends to be opportunistic in the development of products by exploring a variety of avenues, including co-development through potential collaborations, strategic partnerships or other transactions with third parties. The Corporation may seek additional equity and non-dilutive funding and partnerships to advance the development of its product candidates.
PLATFORM AND PRODUCTS IN DEVELOPMENT
Delivery Platform
The DPX platform is a unique and patented formulation providing a new way to deliver active ingredients to the immune system. It relies on a no release mechanism of action (“MOA”) forcing an active uptake by antigen presenting cells.
IMV is exploiting this MOA to pioneer a new class of immunotherapy that represents a paradigm shift from current approaches. By not releasing the active ingredients at the site of injection, it bypasses the steps involved in conventional immune “native responses” such as vaccines, and enables access and programming of immune cells in-vivo to generate new “synthetic” therapeutic capabilities
The DPX platform is based on active ingredients formulated in lipid nanoparticles and, after freeze drying, suspended directly into oil. DPX-based products are stored in the dry format, which provides the added benefit of an extended shelf life. The formulation is designed to be easy to re-suspend and administer to patients.
The DPX platform forms the basis of all of IMV’s product development programs.
The Corporation believes the novel MOA of DPX makes the platform uniquely suitable for cancer immunotherapies, which are designed to target tumor cells. DPX can induce prolonged target-specific and polyfunctional T cell responses, which are postulated to be required for effective tumor control.
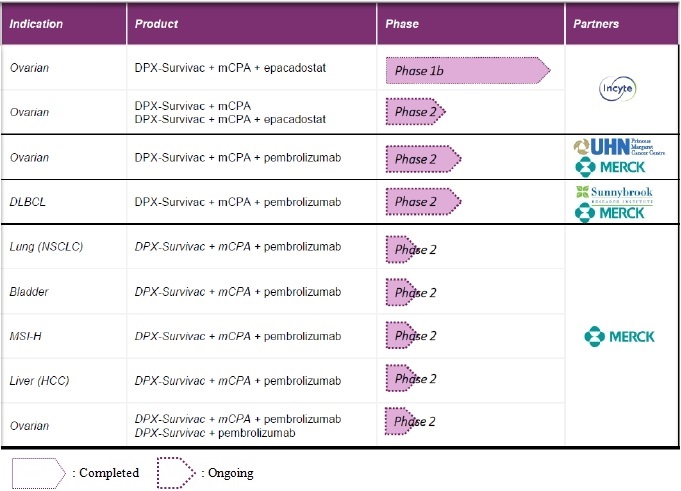
IMV already completed a phase 1 and phase 1b with its lead product candidate, DPX-Survivac, in 56 patients in Ovarian Cancer. Positive results from these first two clinical trials led to a significant expansion of the clinical pipeline of the Corporation, now including eight phase 2 combination trials with partners in six different cancer indications for DPX-Survivac.
5
IMMUNO-ONCOLOGY
DPX-Survivac
Pipeline
Product Overview
DPX-Survivac uses survivin-based peptides licensed from Merck KGaA, on a world-wide exclusive basis, formulated in DPX. Survivin is a major tumor-associated antigen over-expressed in many cancers, making it a viable target for a broadly applicable immunotherapy. DPX delivers the survivin-based antigens in a lipid depot-based format designed to generate a strong and prolonged immune response.
Survivin is essential for the survival of cancer cells and functions as an inhibitor of cell death, known as apoptosis. The presence of high levels of survivin in cancer cells is believed to make them susceptible to a survivin-targeted therapy. The Corporation’s survivin-based therapeutic candidate, DPX-Survivac, aims to train the immune system to recognize and kill survivin-containing cancer cells. This could provide a clinical benefit to patients by reducing tumor burden, delaying cancer progression and/or increasing overall survival. The United States National Cancer Institute has recognized survivin as a promising antigen for cancer treatment based on its specificity, over-expression in cancer cells and immunogenicity potential.
The Corporation believes DPX-Survivac could have broad commercial application as a cancer immunotherapy because it may be applicable for the treatment of multiple solid tumors and hematological cancers, including ovarian, glioblastoma, breast, pancreatic, multiple myeloma, B-cell lymphoma, and melanoma, among other cancers. The Corporation intends to continue the development of DPX-Survivac in a broader range of cancer indications to evaluate additional opportunities.
6
Phase 1b/2 clinical trial in ovarian cancer with Incyte
In June 2015, the Corporation announced it had entered into a non-exclusive clinical trial collaboration with Incyte to evaluate the combination of IMV’s novel T cell activating immunotherapy, DPX-Survivac, with Incyte’s investigational oral IDO1 inhibitor, epacadostat. IMV and Incyte are co-funding and conducting a multicenter, open-label, phase 1b study to evaluate the safety, tolerability and efficacy of the novel combination in platinum resistant or sensitive ovarian cancer patients who are at high risk of recurrence. All patients enrolled in the trial have recurrent ovarian cancer with evidence of progressive disease. The investigational new drug (IND) application for the study, which is testing the triple combination of DPX-Survivac, two doses of epacadostat (100 milligrams and 300 milligrams) and low dose oral cyclophosphamide, was approved by the U.S. Food and Drug Administration (“FDA”) and Health Canada in January 2016. The study was initiated on September 8, 2016 and investigators have now completed enrolment with a total of 53 patients across the two dosing groups, as announced on August 9, 2018.
The Corporation announced, in March 2017, the first interim data analysis from this clinical study. The analysis included the results of blood tests, tumor biopsies and CT scans to assess safety, disease progression and T cell response for the first four evaluable patients in the trial. Based on the interim analysis, the combination therapy appears to have an acceptable tolerable safety profile, with a single grade 3 and single grade 4 event reported and no serious adverse events (“SAEs”). At the time of the interim analysis, three of four patients exhibited stable disease, while a fourth patient progressed and exited the trial. In addition, researchers observed an increased T cell activity in tumors in three of the four patients based on RNA sequencing and indications of early tumor shrinkage in the patient who has been in trial for the longest duration thus far (based on CT scan at day 140).
In December 2017, the Corporation provided positive top-line clinical data. Initial results from 10 evaluable patients in the DPX-Survivac plus-100 milligrams epacadostat dosing cohort demonstrated a disease control rate of 70 per cent. This included partial responses (“PR”, which is defined as equal to 30-per-cent decrease in tumour lesion size) in 30 per cent of the patients (three out of 10). The combination also exhibited a well-tolerated safety profile, with the majority of adverse events (“AEs”) reported as Grade 1 and Grade 2 AE.
Blood tests indicated that the majority of treated patients exhibited targeted T cell activation. Tumour biopsies and analyses thus far have supported the reported MOA of this immunotherapy combination, with DPX-Survivac triggering T cell infiltration into the tumor. This T cell activation was also correlated with tumor regression.
At the time of data cut-off, there were also preliminary data on the first three evaluable patients in the second dosing cohort evaluating the combination of 300 mg BID epacadostat, DPX-Survivac, and low-dose cyclophosphamide. From the first three evaluable patients, two showed stable disease, with one of the two patients showing tumor regression of approximately 25 per cent.
On April 24, 2018, the Corporation announced that it has entered into an agreement with Incyte Corporation to expand their ongoing clinical trial collaboration. The Companies plan to add a phase 2 component to their ongoing Phase 1b combination study evaluating the safety and efficacy of IMV’s lead candidate, DXP-Survivac, in combination with Incyte’s IDO1 enzyme inhibitor epacadostat and low dose cyclophosphamide in advanced ovarian cancer patients.
The phase 2 component is a randomized, open label, efficacy study that will include up to 32 additional evaluable subjects. It will evaluate DPX-Survivac and low dose cyclophosphamide with, and without, epacadostat in patients with advanced recurrent ovarian cancer. In accordance with regulatory guidelines for combination trials, the goal of this part of the program is to evaluate the clinical contribution of each investigational drug in the combination regimen.
The phase 2 arm of the study is conducted under an amendment to the existing collaboration, in which IMV and Incyte are co-funding the trial, and on August 9, 2018, the Corporation announced that the first patient had been treated.
At the American Society for Clinical Oncology June 2018 meeting, IMV provided an update on the clinical trial. At the time of data cut-off, 39 patients were enrolled (including 25 new participants in the 300mg cohort with 8 evaluable from day 56 first CT scan). Data from the first 18 evaluable patients across both dosing cohorts showed:
7 tumor regressions, including 4 PR reported so far; and
Study participants were generally tolerating treatments well, with no related SAEs reported.
Data from the first 8 evaluable participants in the 300mg epacadostat dosing cohort at first CT scan included:
6 patients demonstrated stable disease (“SD”) at day 56, with 4 of these SDs still on trial at data cut-off; and
7
2 patients with tumor regressions observed so far, including one PR with a tumor regression ongoing for more than 9 months.
IMV plans to report updated results on these patients and others enrolled in the trial when data from at least 16 evaluable participants in the second dosing cohort are available.
Researchers also analyzed patient data to study the combination’s MOA. They examined blood samples and tumor biopsies for the 10 evaluable patients treated in the first dosing cohort. These data showed:
Survivin-specific T cell responses detected in 100% (10/10) of patients;
Increase in T cell infiltration post treatment in 37% (3/8) of the analyzable tumor biopsies based on two complementary testing methodologies (RNA sequencing and immunohistochemistry);
2 of the 3 patients with T cell infiltration showed PRs with significant and durable tumor regressions lasting more than one year; and
The third patient with T cell infiltration exhibited Progressive Disease with evidence of down regulation of the major histocompatibility presentation pathway and significant increases in suppressive markers, both indicative of mechanisms of resistance.
The Corporation has requested a type B meeting with the FDA to discuss the possibility of conducting a registration trial in ovarian cancer. At this stage, it is not possible to determine if the FDA would agree; and, if they agree, what type of clinical trial design would be requested and what its cost would be.
The Corporation currently anticipates that, in addition to general clinical expenses which are distributed amongst its various clinical projects, its share of the cost (50%) for 2018 will be approximately $1,300,000, and a total of $1,500,000 in 2019 and 2020 will be required to complete the phase 1b/2 clinical trial with Incyte.
Phase 2 clinical trial in ovarian cancer with Merck (investigator-sponsored)
In February 2017, the Corporation announced an Investigator-Sponsored phase 2 clinical trial in ovarian cancer in combination with Merck’s checkpoint inhibitor pembrolizumab in patients with recurrent, platinum-resistant ovarian cancer. University Health Network’s (“UHN”) Princess Margaret Cancer Centre will conduct the phase 2 non-randomized, open-label trial designed to evaluate the potential anti-tumor activity of the combination of pembrolizumab, DPX-Survivac, and low-dose cyclophosphamide. It is expected to enroll 42 subjects with advanced epithelial ovarian, fallopian tube or primary peritoneal cancer. The study’s primary objective is to assess overall response rate. Secondary study objectives include progression free survival rate, overall survival rate, and potential side effects, over a five-year period. At this stage, the Corporation has no specific plan on the next steps after this trial as it will have to be assessed with its partner based on the clinical trial results.
The Corporation expects to disclose preliminary results in 2018 once provided by the UHN Princess Margaret Cancer Centre and currently anticipates that, in addition to general clinical expenses which are distributed amongst the various clinical projects, its share of the costs to complete this study, that are expected to be spent in 2018, will be approximately $400,000.
Phase 2 clinical trial in Diffuse large B-cell lymphoma (“DLBCL”) with Merck (investigator-sponsored)
On November 8, 2017, the Corporation announced that Health Canada had granted Sunnybrook Research Institute regulatory clearance to begin recruiting patients for its phase 2 clinical study of a triple-combination immunotherapy in patients with measurable or recurrent diffuse large B-cell lymphoma. This trial, announced initially in May 2017, is designed to evaluate the safety and efficacy of IMV’s lead product candidate, DPX-Survivac, along with Merck’s pembrolizumab and low-dose cyclophosphamide in this patient population. On March 28, 2018, the Corporation announced that the first patient had been treated.
Researchers conducting the investigator sponsored study are testing the novel immunotherapy combination in patients whose DLBCL expresses survivin, a tumor antigen highly expressed in 60 percent of DLBCL patients. DPX Survivac stimulates the immune system to produce T cell responses targeting survivin. The non-randomized, open label study is expected to enroll 25 evaluable participants at five centers in Canada. At this stage, the Corporation has no specific plan with respect to the next steps after this trial as it will have to be discussed with its partner based on the outcome of clinical results.
8
On September 18, 2018, IMV announced details of the initial data from this clinical trial. The preliminary data included assessment of safety and clinical activity (based on modified Cheson criteriai) for the first four evaluable patients who have completed their first CT scan after the start of treatment. The data showed that:
Two of the first four evaluable participants showed tumor regressions at the first on-treatment CT scan:
The first enrolled participant demonstrated a tumor regression of 48% at first on-treatment scan; and
The second participant demonstrated a partial response (PR) via a tumor regression of 66% at first on-treatment scan.
Preliminary data from the third participant demonstrated stable disease.
The other participant had early disease progression less than two months following treatment initiation and was discontinued from the study.
The combination therapy appears to demonstrate an acceptable safety profile, with no serious adverse events reported to date.
i Cheson, B.D.,, Pfistner, B., Juweid, M.E., Gascoyne, R.D., Specht, L., Horning, S.J. and Diehl, V. (2007). Revised Response Criteria for Malignant Lymphoma. Journal of Clinical Oncology, 25(5) DOI: 10.1200/JCO.2006.09.2403
The Corporation expects to disclose top-line results around the end of 2018 or in early 2019 once provided by the investigator. The Corporation currently anticipates that, in addition to general clinical expenses which are distributed amongst the various clinical projects, its share of the cost to complete this study will be approximately $2,800,000, of which $1,000,000 is expected to be spent in 2018.
Phase 2 basket trial in 5 indications with Merck
On September 11, 2018, the Corporation announced the expansion of its clinical program with a Phase 2 basket trial in collaboration with Merck evaluating its lead candidate, DPX-Survivac, in combination with low dose cyclophosphamide and Merck’s anti-PD-1 therapy, KEYTRUDA® (pembrolizumab) in patients with select advanced or recurrent solid tumors.
The open-label, multicenter, Phase 2 basket study will evaluate the safety and efficacy of the immunotherapeutic combination agents in patients with bladder, liver (hepatocellular carcinoma), ovarian, or non-small cell lung (NSCLC) cancers as well as tumors shown to be positive for the microsatellite instability high (MSI-H) biomarker. Investigators plan to enroll more than 200 patients across five indications at multiple medical centers in Canada and the United States. IMV expects to initiate trial enrollment in the 4th quarter of 2018.
The American Society of Clinical Oncology (ASCO) defines a basket clinical study as a trial that investigates the effects of a drug regimen in multiple tumor types that share a common molecular target, regardless of where the disease originated.
This is the third clinical trial evaluating the combination of DPX-Survivac, low dose cyclophosphamide, and pembrolizumab in advanced recurrent cancers.
The Corporation currently anticipates that, in addition to general clinical expenses which are distributed amongst the various clinical projects, $3,600,000 is expected to be spent in 2018 and $5,000,000 in 2019 for the safety lead-in for this trial.
Orphan Drug Status and Fast Track Designation
The Corporation announced, in November 2016, that the European Medicines Agency (EMA) had granted orphan drug designation status to IMV’s DPX-Survivac in ovarian cancer. In July 2015, the FDA also granted orphan drug status to DPX-Survivac for the treatment of ovarian cancer. This designation is valid for all applications of DPX-Survivac in ovarian cancer without restriction to a specific stage of disease.
IMV had previously received FDA fast track designation for DPX-Survivac. The designation is intended for patients with no measurable disease after their initial surgery and chemotherapy.
9
Other Programs
In collaboration with commercial and academic partners, the Corporation is also expanding the application of DPX as a delivery platform. Pre-clinical and clinical studies have indicated that the DPX platform may allow for the development of a wide range of applications by generating a stronger and more durable immune activation. The Corporation’s goal is to expand new applications of the DPX platform on its own and with partners.
HPV related cancers
On April 17, 2017, the Corporation announced that the first study participant had been treated in a phase 1b/2 clinical study evaluating IMV’s investigational cancer vaccine, DPX-E7, in combination with low-dose cyclophosphamide in patients with incurable oropharyngeal, cervical and anal cancers related to HPV.
Dana-Farber is leading the DPX-E7 study through a $1.5 million research grant from Stand Up To Cancer and the Farrah Fawcett Foundation to clinically evaluate collaborative translational research that addresses critical problems in HPV-related cancers.
The Dana-Farber study is a single center, open label, non-randomized clinical trial that will investigate the safety and clinical efficacy of DPX-E7 in combination with low-dose metronomic oral cyclophosphamide in a total of 44 treated participants. Its primary objectives are to evaluate changes in CD8+ T cells in peripheral blood and tumor tissue, and to evaluate the safety of DPX-E7 vaccination in HLA-A2 positive patients with incurable HPV-related head and neck, cervical or anal cancers. DPX-E7 targets an HPV viral protein known as E7. IMV has the option to produce the DPX-E7 vaccine if it proves successful in the clinical trials.
The Corporation expects to disclose preliminary results when provided by Dana-Farber.
RSV
Product Overview
A component of the Corporation’s business strategy is partnering the DPX platform within infectious and other diseases. The DPX platform has the potential to generate a rapid and robust immune response, often in a single dose. The unique vaccine enhancement and single-dose capability could prove to be beneficial in targeting difficult infectious and other disease candidates.
The Corporation has performed pre-clinical research activities for a vaccine targeting RSV, which is the second leading cause of respiratory illness in infants, the elderly and the immunosuppressed. Currently, there is no vaccine available for this virus and IMV is seeking to develop a novel vaccine formulation to be used in elderly and healthy adults, including women of child-bearing age. IMV has in-licensed the RSV antigen exclusively from VIB, a non-profit life sciences research institute funded by the Flemish government, to expand its pipeline of vaccine candidates. The novel RSV antigen being evaluated in DPX is based on the short hydrophobic protein present at low levels on the surface of the RSV virion but more importantly also present on the surface of RSV-infected cells. This vaccine has a unique MOA, in that the resultant antibodies bind to and destroy infected cells rather than directly bind to and neutralize free virus.
Phase 1 clinical trial in RSV
A phase 1 clinical study has been conducted in Canada with the Corporation’s RSV vaccine in healthy adults. The RSV vaccine is formulated in IMV’s proprietary DPX platform and is initially being developed to protect the elderly population from infection. The phase 1 study, which was the first clinical trial of a DPX-based vaccine in an infectious disease indication, has evaluated the safety and immune response profile of the RSV vaccine candidate in 40 healthy older adult volunteers (age 50-64 years) and two dose cohorts, with 20 subjects in each cohort.
In July 2016, the Corporation announced positive interim results from this trial. Investigators analyzed the safety and immune response data of all participants up to study day 84. The safety analysis indicates that DPX-RSV was well tolerated among all study participants, with no SAEs recorded. Furthermore, immunogenicity data supported DPX-RSV’s ability to generate a relevant immune response; the vaccine candidate obtained antigen-specific antibody responses in 75 percent of subjects vaccinated with the lower dose and 100 percent of those vaccinated with the higher dose.
In October 2016, the Corporation announced positive topline results from this trial. The report outlined that more than nine months after the last vaccination, 15 of 16 participants (93%) who received DPX-RSV demonstrated antigen-specific immune responses.
10
The vaccine candidate also continued to have a positive safety profile and was well tolerated with no SAEs among all study participants.
On April 12, 2017, the Corporation announced additional positive data from an extended evaluation of patients in this trial. An amendment had been submitted to Health Canada to test subjects who received the higher dose of vaccine out to one year after the booster vaccination. In the 25µg dose cohort, which was the only dose tested out to one year, 100 percent of older adults (7/7 immune responders) vaccinated with DPX-RSV maintained the antigen-specific immune responses one year after receiving the booster dose. At one year, the antibody levels measured were still at peak with no sign of decrease.
On September 27, 2018, IMV announced results of ongoing research to further explore the novel MOA of its vaccine candidate. New data from a preclinical study highlighted the effects of two potential approaches to preventing RSV, comparing a single dose bovine version of DPX-RSV to a two-dose conventional investigational bovine RSV vaccine. Researchers found that IMV’s vaccine candidate yielded strong antigen-specific immune responses and a protective effect on disease pathology. The degree of protection was comparable between the two vaccine candidates.
In this study, researchers compared the effects of both the IMV and conventional RSV vaccine approaches among bovines with known RSV infections (the bovine animal model is considered an optimal model of RSV infection). Researchers administered one dose of DPX-bRSV to one cohort; the second received two doses of a subunit RSV bovine vaccine. Researchers measured immune response with an antibody titer test, and assessed disease pathology with a lung lesion score and other clinical parameters (such as body temperature changes).
They found SH antibodies in 14 of the 15 subjects that received DPX-bRSV, and the improvements observed in disease pathology were comparable between the two cohorts. These were the first bovine animal health data to directly correlate the vaccine-induced immune response against IMV’s novel RSV target - the SH viral protein- with measures of disease protection.
Conventional RSV vaccine candidates target either the F or G proteins of the virus providing protection by neutralizing the RSV virus. Clinical measures of efficacy focus on the amount of neutralizing antibodies in the bloodstream. DPX-RSV works differently; it targets the SH viral ectodomain of the RSV virus, and, instead of neutralizing the virus, it enables the immune system to recognize and destroy infected cells. Because there are no neutralizing antibodies resulting from the DPX-RSV MOA, a different clinical assessment is required to determine the vaccine candidate’s protective effect. IMV has exclusive worldwide licenses on applications that target the SH ectodomain antigen in RSV. The Corporation intends to explore opportunities to out-license this product to potential partners.
Malaria
In 2016, IMV was awarded a subcontract by Leidos, a health, national security, and infrastructure solutions company, to evaluate IMV’s DPX™ platform for the development of peptide-based malaria vaccine targets. The subcontract is funded through Leidos’ prime contract from the U.S. Agency for International Development (“USAID”) to provide vaccine evaluations in the preclinical, clinical and field stages of malaria vaccine development.
In November 2017, an expansion of this collaboration was announced. Following the achievement of several preclinical milestones in the collaboration with USAID, Leidos and USAID selected the DPX-based platform as one of the preferred formulations for further development under a new contract extension. Under the new subcontract, the collaborators will conduct additional research that focuses on identifying the most promising target-formulation combinations.
Zoetis collaboration
In August 2017, the Corporation announced the achievement of several milestones in its ongoing collaboration with global animal health company Zoetis to develop cattle vaccines. In recent controlled studies, the IMV formulations met efficacy and duration of immunity end-points against two disease targets. These results will enable Zoetis to advance two IMV-formulated vaccine candidates into late-stage testing.
Licensing Agreements
While the Corporation is focused on developing a pipeline of cancer immunotherapies, it is also pursuing opportunities to license the Corporation’s platform technology to other parties interested on an application-by-application basis.
11
In April 2018, IMV signed a licensing agreement and granted SpayVac-for-Wildlife (SFW Inc.) a license to two of its proprietary delivery platforms. SFW Inc. has global exclusive rights to use both of these platforms to develop humane, immuno-contraceptive vaccines for control of overabundant, feral and invasive wildlife populations against royalties on sales.
MARKET OVERVIEW
Cancer Immunotherapies
Cancer is considered one of the most widespread and prevalent diseases globally. According to Global Cancer Facts & Figures, 3rd edition (released February 2015 by the American Cancer Society), it is predicted that new cancer cases will rise to 21.7 million and the number of cancer deaths to 13 million by 2030. Conventional cancer treatment involves surgery to remove the tumor when possible, as well as chemotherapy and radiation. Chemotherapies are widely used despite their associated toxicities because they interfere with the ability of cancer cells to grow and spread. However, tumors often develop resistance to chemotherapies, limiting their efficacy in preventing tumor recurrence. Despite recent advances, independent sources note a high unmet medical need in cancer therapy, noting the median survival rate remains poor. Cancer immunotherapies, including therapeutic cancer vaccines, may provide a new and effective treatment. According to a Market & Markets report released in January 2017, the global immunotherapy drugs market is projected to reach USD $201.52 billion by 2021 from USD $108.41 billion in 2016, growing at a compound annual growth rate of 13.5% during the forecast period of 2016 to 2021. The major players operating in the immunotherapy drugs market include F. Hoffmann-La Roche AG (Switzerland), GlaxoSmithKline (U.K.), AbbVie, Inc. (U.S.), Amgen, Inc. (U.S.), Merck & Co., Inc. (U.S.), Bristol-Myers Squibb (U.S.), Novartis International AG (Switzerland), Eli Lilly and Corporation (U.S.), Johnson & Johnson (U.S.), and AstraZeneca plc (U.K.).
Cancer immunotherapy seeks to harness the immune system to assist in the destruction of tumors and to prevent their recurrence. There has been significant interest in the field of cancer immunotherapy stemming from recent clinical success in prolonging patient survival with novel compounds. The ability to apply these appropriately has resulted from a greater understanding of the immune dysfunction that is characteristic of cancer. One area in which there have been breakthroughs has been in the area of checkpoint inhibitors, compounds that target key regulatory molecules of the immune system. Yervoy (anti-CTLA-4, or ipilumumab, developed by Bristol-Myers Squibb) was the first compound in this class to be approved for use in advanced metastatic melanoma. In cancer, these regulators (CTLA-4, PD-1 and its ligand PD-L1) act to inhibit CD8 T cell mediated anti-tumor immune responses that are crucial for tumor control. Monoclonal antibodies that target PD-1 and PD-L1 have shown unusual efficacy in cancer patients, with a significant percentage of patients experiencing durable response to these therapies. Several of these compounds are in advanced clinical trials, with one compound, Merck’s Keytruda (pembrolizumab), having received FDA approval in September 2014 for advanced melanoma patients who have stopped responding to other therapies. Bristol-Myers Squibb’s compound nivolumab (Opdivo) has also been approved in the United States and Japan. These therapies have recently been approved for use in other advanced cancers including bladder cancer, non-small cell lung cancer, Hodgkin’s Lymphoma, squamous cell carcinoma of the head and neck and stomach cancer. In addition, Keytruda in particular has been approved for use in cancers with a specific molecular indication irrelevant of cancer type. Keytruda was also approved in May for use to treat solid tumors having a biomarker for microsatellite instability (MSI-H), which is a defect in the DNA repair pathway. This represents about 5% of a number of different tumor types, including colorectal, breast, prostate and thyroid cancers.
Key opinion leaders in the field have indicated that the ideal combination, with checkpoint inhibitors, is likely to be a therapy that drives tumor specific immune responses. These include novel cancer vaccines and T cell-based therapies. These therapies fit well with checkpoint inhibition therapy because they simultaneously activate strong tumor specific immune responses, while releasing the brakes on immune suppression. The success of such combinations should allow pharmaceutical companies to significantly expand the market of their checkpoint inhibitors, which are currently effective in approximately 10% to 30% of patients.
The Corporation believes that T cell therapies will become an important component of these novel combination immunotherapies, with the potential of synergistic benefits to become an essential part of a multi-pronged approach for the treatment of cancer.
INTELLECTUAL PROPERTY
The Corporation strives to protect its intellectual property in established, as well as emerging, markets around the world. The Corporation’s intellectual property portfolio relating to its vaccine platform technology includes sixteen patent families, the first of which contains eight patents issued in five jurisdictions (United States, Europe, Canada, Japan and Australia). The fifteen other families collectively contain thirty-seven patents issued in ten jurisdictions (United States, Europe, Canada, Australia, Japan, India, Israel, Singapore, China and separately Hong Kong) and fifty pending patent applications in eleven jurisdictions. Taking into accounts the validations of the European patents, the Corporation’s intellectual property portfolio includes seventy-five patents. More details on the Corporation intellectual property strategy and patents can be found in the AIF filed on SEDAR at www.sedar.com.
12
The Corporation owns registered trademarks in the United States, Canada and Europe.
RECENT AND QUARTERLY DEVELOPMENTS
Key developments and achievements
The Corporation announced:
On September 27, 2018, results of ongoing research to further explore the novel MOA of its vaccine candidate. New data from a preclinical study highlighted the effects of two potential approaches to preventing RSV, comparing a single dose bovine version of DPX-RSV to a two-dose conventional investigational bovine RSV vaccine. Researchers found that IMV’s vaccine candidate yielded strong antigen-specific immune responses and a protective effect on disease pathology.
The degree of protection was comparable between the two vaccine candidates.
In this study, researchers compared the effects of both the IMV and conventional RSV vaccine approaches among bovines with known RSV infections (the bovine animal model is considered an optimal model of RSV infection). Researchers administered one dose of DPX-bRSV to one cohort; the second received two doses of a subunit RSV bovine vaccine. Researchers measured immune response with an antibody titer test, and assessed disease pathology with a lung lesion score and other clinical parameters (such as body temperature changes).
They found SH antibodies in 14 of the 15 animals that received DPX-bRSV, and the improvements observed in disease pathology were comparable between the two cohorts. These were the first bovine animal health data to directly correlate the vaccine-induced immune response against IMV’s novel RSV target - the SH viral protein- with measures of disease protection.
On September 18, 2018, details of the initial data from its ongoing investigator-sponsored Phase 2 clinical trial in DLBCL. In the study, investigators are evaluating IMV’s lead candidate, DPX-Survivac, in combination with low dose cyclophosphamide and Merck’s checkpoint inhibitor Keytruda® (pembrolizumab), in patients with persistent or recurrent/refractory DLBCL.
The preliminary data included assessment of safety and clinical activity (based on modified Cheson criteriai) for the first four evaluable patients who have completed their first CT scan after the start of treatment. The data showed that:
Two of the first four evaluable participants showed tumor regressions at the first on-treatment CT scan:
The first enrolled participant demonstrated a tumor regression of 48% at first on-treatment scan; and
The second participant demonstrated a partial response (PR) via a tumor regression of 66% at first on- treatment scan.
Preliminary data from the third participant demonstrated stable disease.
The other participant had early disease progression less than two months following treatment initiation and was discontinued from the study.
The combination therapy appears to demonstrate an acceptable safety profile, with no serious adverse events reported to date.
i Cheson, B.D.,, Pfistner, B., Juweid, M.E., Gascoyne, R.D., Specht, L., Horning, S.J. and Diehl, V. (2007). Revised Response Criteria for Malignant Lymphoma. Journal of Clinical Oncology, 25(5) DOI: 10.1200/JCO.2006.09.2403
On September 11, 2018, an expansion of its clinical program with a phase 2 basket trial in collaboration with Merck evaluating its lead candidate, DPX-Survivac, in combination with low-dose cyclophosphamide and Merck’s anti-PD-1 therapy, Keytruda (pembrolizumab), in patients with select advanced or recurrent solid tumours across five indications.
The open-label, multicentre, phase 2 basket study will evaluate the safety and efficacy of the immunotherapeutic combination agents in patients with bladder, liver (hepatocellular carcinoma), ovarian or non-small-cell lung (NSCLC) cancers, as well as tumours shown to be positive for the microsatellite instability high (MSI-H) biomarker. Investigators plan to enroll more than 200 patients across five indications at multiple medical centres in Canada and the United States. IMV expects to initiate enrolment for this trial in the fourth quarter of 2018.
On August 9, 2018, IMV reached two important milestones in its continuing clinical trial collaboration with Incyte Corp. Investigators have completed enrolment for both phase 1b dosing cohorts and have treated the first patient in the phase 2 component of the combination trial, which is evaluating the safety and efficacy of IMV’s lead candidate, DPX-Survivac, and low-dose cyclophosphamide with (and without) epacadostat in patients with advanced ovarian cancer.
13
Investigators have completed enrolment in the phase 1b cohorts of the study, with a total of 50 patients across the two dosing groups. The phase 1b study is evaluating the safety and efficacy of combining DPX-Survivac, 100 milligrams or 300 milligrams of epacadostat, and low-dose cyclophosphamide in individuals with advanced, platinum-sensitive and resistant ovarian cancer.
SELECTED FINANCIAL INFORMATION
| Three months | Three months | Nine months | Nine months | |
| ended | ended | ended | ended | |
| September 30, | September 30, | September 30, | September 30, | |
| 2018 | 2017 | 2018 | 2017 | |
| $ | $ | $ | $ | |
| Loss for the period | (5,987,000) | (2,122,000) | (14,254,000) | (7,099,000) |
| Basic and diluted loss per share | (0.14) | (0.06) | (0.33) | (0.18) |
| As at | As at | |
| September 30, 2018 | December 31, 2017 | |
| $ | $ | |
| Cash and cash equivalents | 20,271,000 | 14,909,000 |
| Total assets | 26,213,000 | 17,032,000 |
| Lease obligations | 1,332,000 | - |
| Long term debt | 7,401,000 | 6,476,000 |
RESULTS FOR THE THREE AND NINE MONTHS ENDED SEPTEMBER 30, 2018, COMPARED TO THE THREE AND NINE MONTHS ENDED SEPTEMBER 30, 2017
| Nine | Nine | |||
| Months | Months | |||
| Q3 2018 | Q3 2017 | ended | ended | |
| September | September | |||
| 30, 2018 | 30, 2017 | |||
| $ | $ | $ | $ | |
| Revenue | 125,000 | 53,000 | 349,000 | 123,000 |
| Research and development | 3,897,000 | 1,341,000 | 8,384,000 | 3,610,000 |
| General and administrative | 1,923,000 | 942,000 | 4,892,000 | 2,833,000 |
| Business development and investor relations | 426,000 | 237,000 | 1,389,000 | 963,000 |
| Government assistance | (404,000) | (624,000) | (868,000) | (1,003,000) |
| Accreted interest | 270,000 | 279,000 | 806,000 | 819,000 |
| Net loss and comprehensive loss for the period | (5,987,000) | (2,122,000) | (14,254,000) | (7,099,000) |
Revenue
Revenue increased by $72,000 in Q3 2018 and $226,000 for the first nine-months of 2018 in comparison with the corresponding periods in 2017. Interest revenue increased by $66,000 in Q3 2018 and $177,000 for the first nine-months of 2018 compared to 2017 explained by higher cash balances since the beginning of 2018. The remainder of the increase during the quarter and, since the beginning of 2018, is attributable to an increase in subcontract revenue.
14
Operating expenses
Overall operating expenses increased by $3,937,000 to $6,112,000 during Q3 2018 compared to Q3 2017 and by $7,381,000 since the beginning of 2018. Explanations of the nature of costs incurred, along with explanations for those changes in costs are discussed below:
Research and development expenses
R&D expenses include salaries and benefits, expenses associated with the phase 1b and phase 2 clinical trials of DPX-Survivac, clinical research and manufacturing of DPX-RSV and DPX-Survivac, consulting fees paid to various independent contractors with specific expertise required by the Corporation, the cost of animal care facilities, laboratory supplies, peptides and other chemicals, rental of laboratory facilities, insurance, as well as other R&D related expenses.
The Corporation’s R&D efforts and related expenses for Q3 2018 and for the nine-months of 2018 included costs surrounding the Corporation’s clinical trials of DPX-Survivac, namely the phase 1b/2 clinical trial collaboration with Incyte in ovarian cancer, Phase 2 clinical trial collaboration with Merck in ovarian cancer, phase 2 clinical trial collaboration with Merck in DLBCL, basket trial start up costs and costs related to the Corporation’s ongoing R&D activities associated with the investigation, and analysis and evaluation of other potential product candidates and technologies.
Research and development expenses consist of the following:
| Nine Months | Nine Months | |||
| Q3 2018 | Q3 2017 | Ended | Ended | |
| September | September | |||
| 30, 2018 | 30, 2017 | |||
| $ | $ | $ | $ | |
| General research and development expenses | 678,000 | 397,000 | 1,613,000 | 1,153,000 |
| DPX-Survivac preclinical and clinical expenses | 2,214,000 | 338,000 | 4,171,000 | 778,000 |
| Salaries and benefits | 872,000 | 545,000 | 2,230,000 | 1,461,000 |
| Stock-based compensation | 103,000 | 35,000 | 291,000 | 162,000 |
| Depreciation | 30,000 | 26,000 | 79,000 | 56,000 |
| Total | 3,897,000 | 1,341,000 | 8,384,000 | 3,610,000 |
The increase in general R&D expenses from $397,000 for Q3 2017 to $678,000 in Q3 2018 is mainly attributable to a $196,000 increase in regulatory consulting, a $30,000 increase in raw materials and supplies and a $37,000 increase in professional development. Since the beginning of the year, the increase of $460,000 is mainly explained by a $200,000 increase in regulatory consulting, a $144,000 increase in professional fees and consulting for analysis of clinical results and a $111,000 increase in R&D travel and conferences.
The increase of $1,885,000 in Q3 2018 and $3,393,000 since the beginning of 2018 in DPX-Survivac preclinical and clinical expenses is mainly attributable to increased clinical activity including: higher enrollment in the phase 1b/2 Incyte trial in ovarian cancer compared with 2017; milestone payments for phase 2 study in DLBCL and phase 2 study in ovarian cancer; and expenses related to the preparation for the beginning of basket trial. The increase is also attributable to manufacturing activities to support the increased clinical activity including purchasing of raw materials and contract manufacturing organization costs.
The increase in R&D salaries in 2018 is mainly attributable to the hiring of new employees in the second half in 2017 and since the beginning of 2018.
15
General and administrative expenses
G&A expenses consist of the following:
| Nine | Nine | |||
| Months | Months | |||
| Q3 2018 | Q3 2017 | Ended | Ended | |
| September | September | |||
| 30, 2018 | 30, 2017 | |||
| $ | $ | $ | $ | |
| General and administrative expenses, excluding salaries | 980,000 | 473,000 | 2,778,000 | 1,200,000 |
| Salaries and benefits | 386,000 | 299,000 | 1,165,000 | 844,000 |
| Stock-based and deferred share unit compensation | 482,000 | 155,000 | 823,000 | 752,000 |
| Depreciation | 75,000 | 17,000 | 126,000 | 37,000 |
| Total | 1,923,000 | 942,000 | 4,892,000 | 2,833,000 |
For Q3 2018, G&A expenses, excluding salaries, increased by $508,000. This is mainly explained by an increase of insurance premium of $157,000 mainly related to the Nasdaq listing, a $87,000 increase in legal fees, a $52,000 increase in travel, a $86,000 increase related to relocating to the new facility and an increase of $45,000 in professional and regulatory fees. Since the beginning of the year, G&A expenses, excluding salaries, increased by $1,578,000 mainly explained by: the various non-recurring expenses of $542,000 related to the Nasdaq listing; $105,000 related to the new facility relocation; the share consolidation and the filing of a shelf prospectus; increase in patent legal expenses of $110,000; increase in other legal expenses of $73,000; increase in consulting and professional fees of $194,000 related mainly to benchmarking and recruiting; an increase of $74,000 in regulatory fees; and a $220,000 increase in insurance premium following the Nasdaq listing.
Salaries and benefits increased by $87,000 in Q3 2018, and $321,000 since the beginning of 2018, due to an overall increase in compensation for the senior executive team, the fact that the CFO was there for the entire nine months in 2018 compared to seven months in 2017, and other hiring in the second half of 2017 and since the beginning of 2018.
The increase in stock-based and deferred share unit compensation in Q3 2018 is explained by an increase of $105,000 in stock-based compensation as more stock options vested in Q3 2018 compared to Q3 2017 and an increase of $222,000 in deferred share units (“DSU”) compensation. The increase in DSU compensation is mainly attributable to the increase in the fair value of the DSUs outstanding since the end of Q2 2018 as well as new DSUs issued during the quarter. The Corporation values its DSU obligation at the current market value of a corresponding number of IMV Inc. common shares and records any fluctuation its the DSU obligation as an expense on the consolidated statements of loss and comprehensive loss.
Government assistance
Government assistance consists of the following:
| Nine | Nine | |||
| Months | Months | |||
| Q3 Fiscal | Q3 Fiscal | Ended | Ended | |
| 2018 | 2017 | September | September | |
| 30, 2018 | 30, 2017 | |||
| $ | $ | $ | $ | |
| Investment tax credits (“ITC”) | (396,000) | (113,000) | (836,000) | (472,000) |
| Government loans and assistance | (8,000) | (511,000) | (32,000) | (531,000) |
| Total | (404,000) | (624,000) | (868,000) | (1,003,000) |
The increase in investment tax credit in Q3 2018 and since the beginning of 2018 is explained by the increase in R&D salaries as well as increased clinical trial activity being performed in Canada. The decrease in government loans and assistance is explained
16
by a $506,000 revaluation of the low-interest bearing government loan from the Province of Nova Scotia upon the receipt of the two-year extension in Q3 2017.
Business development and investor relations expenses
The Corporation’s business development and investor relations activities increased in Q3 2018 by $189,000, compared to Q3 2017, to a total of $426,000. This variation is mainly explained by a $100,000 and $47,000 increase in salary and benefits and stock-based compensation, respectively, relating to the hiring of a Senior Vice President, Business Development in January 2018. The increase of $426,000 in business development and investor relations since the beginning of the year is also mainly explained by this hiring. Salary and benefits and stock-based compensation, respectively, increased by $277,000 and $126,000 during the first nine months of 2018.
Accreted Interest
Accreted interest relates entirely to the valuation of low-interest bearing government loans which are repayable based on a percentage of future gross revenue and is comparable to 2017.
Net loss and comprehensive loss
The net loss and comprehensive loss was $5,987,000 or $0.14 per basic and diluted share for Q3 2018, $3,875,000 higher than the net loss and comprehensive loss of $2,112,000 or $0.05 per basic and diluted share for Q3 2017. For the nine months ended September 30, 2018, the net loss and comprehensive loss was $14,254,000 or $0.33 per basic and diluted share compared to $7,099,000 or $0.19 per basic and diluted share for the nine months ended September 30, 2017.
CASH FLOWS, LIQUIDITY AND CAPITAL RESOURCES
At September 30, 2018, the Corporation had cash and cash equivalents of $20,271,000 and working capital of $18,486,000, compared to $14,909,000 and $13,627,000, respectively as at December 31, 2017.
Since the Corporation’s inception, operations have been financed through the issuance of equity securities, debt, revenue from licenses, cost recoveries from collaborations, interest income on funds available for investment, government assistance and tax credits.
During the first nine months of 2018, $12,213,000 was used in operating activities. This included the reported net loss of $14,254,000 prior to being decreased for non-cash expenses including DSU compensation, depreciation, accretion of long-term debt and lease obligations, loss on disposal of assets and stock-based compensation. The Corporation had a net decrease of cash of $273,000 as a result of changes in working capital balances.
Sources of cash included: $14,375,000 raised through financing activities less cash issuance costs of $1,148,000; and $4,865,000 through the exercise of stock options and warrants. The Corporation received $896,000 in incentive contributions from its lessor and borrowed $200,000 from its lessor to fund leasehold improvements at the new facility in Dartmouth. The Corporation used $64,000 to repay long-term debt and lease obligations during the period and $97,000 to pay taxes related to DSU redemption.
During the nine-month period ended September 30, 2018, the Corporation purchased equipment and leasehold improvements for ongoing research and operating activities for an aggregate amount of $1,466,000. The Corporation raised $14,000 in proceeds from the sale of used furniture at its former Halifax facility.
The Corporation aims to maintain adequate cash and cash resources to support planned activities which include: the phase 1b/2 combination trial with DPX-Survivac and Incyte’s IDO1 inhibitor epacadostat; the two phase 2 investigator-sponsored combination trials with DPX-Survivac and Merck’s checkpoint inhibitor, pembrolizumab in ovarian cancer and DLBCL; initiation of the basket trial in 5 indications with DPX-Survivac and Merck’s checkpoint inhibitor, pembrolizumab; and other research and development activities, business development efforts, administration costs, and intellectual property maintenance and expansion.
At September 30, 2018, the Corporation had approximately $21.9 million of existing and identified potential sources of cash including:
cash and equivalents of $20.3 million; and
amounts receivable and investment tax credits receivable of $1.6 million.
17
For the first nine months of 2018, the Corporation’s “cash burn rate” (defined as net loss for the period adjusted for operations not involving cash - interest on lease obligation, depreciation, accretion of long-term debt, stock-based compensation and DSU compensation) was $11.9 million. Based on the current business plan and depending on the timing of certain clinical expenses, the Corporation forecasts the cash burn rate to be between $4.0 million to $4.5 million for the last quarter of 2018, as it continues to execute its clinical plan.
It is common for early-stage biotechnology companies to require additional funding to further develop product-candidates until successful commercialization of at least one product candidate. IMV’s product candidates are still in the early-development stage of the product cycle and therefore are not generating revenue to fund operations. The Corporation continuously monitors its liquidity position, the status of its development programs including those of its partners, cash forecasts for completing various stages of development, the potential to license or co-develop each vaccine candidate, and continues to actively pursue alternatives to raise capital, including the sale of its equity securities, debt and non-dilutive funding.
Management believes that its cash resources of $20.2 million and its additional potential cash resources of $1.6 million as at September 30, 2018 will be sufficient to fund operations for the next twelve months while maintaining adequate working capital up to the fourth quarter of 2019. The Corporation continually reassesses the adequacy of its cash resources, evaluating existing clinical trials, research projects and/or potential collaboration opportunities, to determine when and how much additional funding is required.
JUNE 2017 EQUITY OFFERING AND USE OF PROCEEDS
On June 21, 2017, the Corporation completed a public offering, issuing 7,692,308 common shares common shares pre-consolidation (2,403,846 post-consolidation) at a price of $1.30 per share pre-consolidation ($4.16 post-consolidation) for aggregate proceeds of $10,000,000. The Corporation intends to use the net proceeds of this offering for the research and development and clinical advancement of its cancer and infectious disease vaccine candidates and for working capital and general corporate purposes. The table below provides the amount used to date and any variances (except for working capital and general corporate purposes).
| Intended Use of Proceeds | Estimated | Amount | Variances |
| amount | to date | ||
| $ | $ | ||
| phase 2 clinical trial in DLBCL with Merck | 2,400,000 | 1,089,000 | No variances anticipated |
| phase 1 clinical trial for multiple indications | 4,200,000 | 636,000 | No variances anticipated |
FEBRUARY 2018 EQUITY OFFERING AND USE OF PROCEEDS
On February 15, 2018, the Corporation completed a public offering, issuing 7,187,500 common shares pre-consolidation (2,246,094 post-consolidation) at a price of $2.00 per share pre-consolidation ($6.40 post-consolidation) for aggregate proceeds of $14,375,000. The Corporation intends to use the net proceeds of this offering to continue to advance the Corporation’s pipeline and conduct a phase 1 basket trial in up to five indications to be identified, for research and development, working capital, and for general corporate purposes. The table below provides the amount used to date and any variances (except for working capital and general corporate purposes).
| Intended Use of Proceeds | Estimated | Amount | Variances |
| amount | to date | ||
| $ | $ | ||
| Clinical trials in 2019 | 4,800,000 | Nil | No variances anticipated |
| Research & development in 2019 | 5,300,000 | Nil | No variances anticipated |
18
SUMMARY OF QUARTERLY RESULTS
The following consolidated quarterly data was drawn from the audited annual consolidated financial statements and the unaudited interim condensed consolidated financial statements. All values discussed below are rounded to the nearest thousand. The information is reported on an IFRS basis.
| Total Revenue | Total Expenses | Loss | Basic and Diluted | |
| Quarter Ended In | Loss Per Share | |||
| $ | $ | $ | $ | |
| Q3 - September 30, 2018 | 125,000 | 6,112,000 | (5,987,000) | (0.14) |
| Q2 - June 30, 2018 | 129,000 | 5,325,000 | (5,196,000) | (0.12) |
| Q1 - March 31, 2018 | 96,000 | 3,163,000 | (3,067,000) | (0.07) |
| Q4 - December 31, 2017 | 66,000 | 4,997,000 | (4,931,000) | (0.13) |
| Q3 - September 30, 2017 | 53,000 | 2,175,000 | (2,122,000) | (0.06) |
| Q2 - June 30, 2017 | 36,000 | 2,642,000 | (2,606,000) | (0.06) |
| Q1 - March 31, 2017 | 34,000 | 2,403,000 | (2,369,000) | (0.06) |
| Q4 - December 31, 2016 | 21,000 | 3,762,000 | (3,741,000) | (0.13) |
Revenues from quarter to quarter may vary significantly. Revenues are non-recurring by nature and are generated by license agreements as well as contract research agreements. It is also important to note that historical patterns of expenses cannot be taken as an indication of future expenses. The amount and timing of expenses and availability of capital resources vary substantially from quarter to quarter, depending on the level of R&D activities being undertaken at any time and the availability of funding from investors or collaboration partners.
OUTLOOK FOR THE REMAINDER OF 2018
The Corporation has many clinical studies ongoing and expects the following timing to disclose results for the following studies:
| Product/study | Partner | Indication | Type of results | Expected Timing |
| DPX-Survivac - phase 1b/2 |
Incyte | Ovarian cancer | Top line clinical results 300mg cohort |
End-2018 |
| DPX-Survivac - phase 2 |
Merck | Ovarian cancer | Preliminary clinical results |
End - 2018 |
The exact timing of disclosure of the above results could differ from our expectations but are currently management’s best estimate.
RELATED PARTY TRANSACTIONS
During Q3 2018, there were no related party transactions (Q3 2017 - $nil).
CONTRACTUAL OBLIGATIONS
As of September 30, 2018, there is no material change in the contractual obligations of the Corporation since the beginning of the 2018 fiscal year. Details on the contractual obligations of the Corporation can be found in the in the audited annual consolidated financial statements and related notes for the year ended December 31, 2017.
19
OFF-BALANCE SHEET ARRANGEMENTS
The Corporation was not party to any off-balance sheet arrangements as of September 30, 2018.
OUTSTANDING SECURITIES
As of November 1, 2018, the number of issued and outstanding common shares was 44,999,802 and a total of 1,951,842 stock options, warrants, and deferred share units were outstanding.
RISKS AND UNCERTAINTIES
The Corporation is a clinical-stage company that operates in an industry that is dependent on a number of factors that include the capacity to raise additional capital on reasonable terms, obtain positive results of clinical trials - including clinical trials on DPX-Survivac, obtain positive results of clinical trials without serious adverse or inappropriate side effects, and obtain market acceptance of its product by physicians, patients, healthcare payers and others in the medical community for commercial success, etc. An investment in the Corporation’s common shares is subject to a number of risks and uncertainties. An investor should carefully consider the risks described in the Corporation’s AIF and the registration statement on Form 40-F filed with the U.S. Securities and Exchange Commission, as well as the other information filed with the securities regulators before investing in the Corporation’s common shares. If any of the such described risks occur, or if others occur, the Corporation’s business, operating results and financial condition could be seriously harmed and investors may lose a significant proportion of their investment.
There are important risks which management believes could impact the Corporation’s business. For information on risks and uncertainties, please also refer to the “Risk Factors” section of our most recent AIF filed on SEDAR at www.sedar.com and included in the registration statement on Form 40-F filed on EDGAR at www.sec.gov/edgar.
DISCLOSURE CONTROLS AND PROCEDURES AND INTERNAL CONTROLS OVER FINANCIAL REPORTING
Disclosure Controls and Procedures
The Chief Executive Officer (the “CEO”) and the Chief Financial Officer (the “CFO”) of the Company are responsible for establishing and maintaining the Company’s disclosure controls and procedures (“DCP”) including adherence to the Disclosure Policy adopted by the Company. The Disclosure Policy requires all staff to keep senior management fully apprised of all material information affecting the Company so that they may evaluate and discuss this information and determine the appropriateness and timing for public disclosure.
The Company maintains DCP designed to ensure that information required to be disclosed in reports filed under applicable securities laws, is recorded, processed, summarized and reported within the appropriate time periods and that such information is accumulated and communicated to the Company’s management, including the CEO and CFO, to allow for timely decisions regarding required disclosure.
The CEO and CFO have evaluated whether there were changes to the DCP during the nine months ended September 30, 2018 that have materially affected, or are reasonably likely to materially affect, the DCP. No such changes were identified through their evaluation.
In designing and evaluating DCP, the Company recognizes that any disclosure controls and procedures, no matter how well conceived or operated, can only provide reasonable, not absolute, assurance that the objectives of the control system are met, and management is required to exercise its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Internal Control over Financial Reporting
The Company’s management, including the CEO and the CFO, are responsible for establishing and maintaining adequate internal control over financial reporting (“ICFR”) for the Company to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with IFRS. The fundamental issue is ensuring all transactions are properly authorized and identified and entered into a well-designed, robust and clearly understood accounting system on a timely basis to minimize risk of inaccuracy, failure to fairly reflect transactions, failure to fairly record transactions necessary to present financial statements in accordance with IFRS, unauthorized receipts and expenditures, or the inability to provide assurance that unauthorized acquisitions or dispositions of assets can be detected.
20
The CEO and CFO have evaluated whether there were changes to the ICFR during the three months ended September 30, 2018 that have materially affected, or are reasonably likely to materially affect, the ICFR. No such changes were identified through their evaluation.
The Company’s ICFR may not prevent or detect all misstatements because of inherent limitations. Additionally, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because changes in conditions or deterioration in the degree of compliance with the Company’s policies and procedures.
BASIS OF PRESENTATION OF CONSOLIDATED FINANCIAL STATEMENTS AND SIGNIFICANT ACCOUNTING POLICIES
The consolidated financial statements have been prepared in accordance with the IFRS as issued by the IASB. The accounting policies, methods of computation and presentation applied in the consolidated financial statements are consistent with those of previous financial year except for the presentation of government assistance now presented as a separate item in the consolidated statements of loss and comprehensive loss and the interest revenue now presented as part of the revenue. Certain comparative figures have been reclassified to conform the presentation adopted in the current year for government assistance and interest revenue.
The significant accounting policies of IMV are detailed in the notes to the audited consolidated financial statements for the year ended December 31, 2017 filed on SEDAR www.sedar.com and included in the registration statement on Form 40-F filed on EDGAR at www.sec.gov/edgar.
CRITICAL ACCOUNTING ESTIMATES AND JUDGEMENTS
Estimates and assumptions are continually evaluated and are based on historical experience and other factors, including expectations of future events that are believed to be reasonable under the circumstances. The determination of estimates requires the exercise of judgement based on various assumptions and other factors such as historical experience and current and expected economic conditions. Actual results could differ from those estimates.
Critical judgements in applying the Corporation’s accounting policies are detailed in the audited annual consolidated financial statements for the year ended December 31, 2017 filed on SEDAR www.sedar.com and included in the registration statement on Form 40-F filed on EDGAR at www.sec.gov/edgar.
FINANCIAL INSTRUMENTS
Financial instruments are defined as a contractual right or obligation to receive or deliver cash on another financial asset. The Corporation recognizes financial instruments based on their classification. Depending on the financial instrument’s classification, changes in subsequent measurements are recognized in net loss or other comprehensive loss.
A description of the financial instruments, their fair value and risk management is included in the Corporation’s audited annual consolidated financial statements for the year ended December 31, 2017 filed on SEDAR www.sedar.com and included in the registration statement on Form 40-F filed on EDGAR at www.sec.gov/edgar.
| (Signed) Frédéric Ors | (Signed) Pierre Labbé |
| Frédéric Ors | Pierre Labbé |
| Chief Executive Officer | Chief Financial Officer |
November 1, 2018
________________________
21