
Information contained herein is subject to completion or amendment. A registration statement relating to these securities has been filed with the United States Securities Exchange Commission. This preliminary prospectus supplement and the accompanying base shelf prospectus shall not constitute an offer to sell or the solicitation of an offer to buy nor shall there be any sale of these securities in any state of the United States in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state of the United States.
Filed Pursuant to General Instruction II.L of Form F-10
File No. 333-225326
Subject to Completion, dated February 28, 2019
A copy of this preliminary prospectus supplement has been filed with the securities regulatory authorities in the provinces of British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Québec, Nova Scotia and Newfoundland and Labrador but has not yet become final for the purposes of the sale of securities. Information contained in this preliminary prospectus supplement may not be complete and may have to be amended.
No securities regulatory authority has expressed an opinion about these securities and it is an offence to claim otherwise. This prospectus supplement, together with the short form base shelf prospectus dated June 5, 2018 to which it relates, as amended or supplemented, and each document incorporated or deemed to be incorporated by reference in this prospectus supplement and in the short form base shelf prospectus dated June 5, 2018 to which it relates, constitutes a public offering of these securities only in those jurisdictions where they may be lawfully offered for sale and therein only by persons permitted to sell such securities. See “Plan of Distribution”.
Information has been incorporated by reference in this prospectus supplement, and in the short form base shelf prospectus dated June 5, 2018 to which it relates from documents filed with securities commissions or similar authorities in Canada. Copies of the documents incorporated herein by reference may be obtained on request without charge from the Corporate Secretary of IMV Inc. at 130 Eileen Stubbs Avenue, Suite 19, Dartmouth, Nova Scotia, Canada, B3B 2C4 (telephone (902) 492-1819), and are also available electronically at www.sedar.com.
PRELIMINARY PROSPECTUS SUPPLEMENT
TO THE SHORT FORM BASE SHELF PROSPECTUS DATED JUNE 5, 2018
| New Issue |

IMV INC.
$
Common Shares
This prospectus supplement (the “Prospectus Supplement”) to the short form base shelf prospectus of IMV Inc. (“IMV” or the “Corporation”) dated June 5, 2018 (the “Base Shelf Prospectus”) qualifies the distribution (the “Offering”) of common shares (the “Offered Shares”) at a price of $ per Offered Share (the “Offering Price”).
The Offering is being made concurrently in Canada under the terms of this Prospectus Supplement and in the United States under the terms of the Corporation’s registration statement on Form F-10 (File No. 333-225326) (as amended, the “U.S. Registration Statement”) filed with the United States Securities and Exchange Commission (the “SEC”) under the U.S. Securities Act of 1933, as amended (the “U.S. Securities Act”).
All dollar amounts in this Prospectus Supplement are in Canadian dollars, unless otherwise indicated. See”Exchange Rate Information”.
The Offered Shares are issued and sold in Canada by Wells Fargo Securities Canada, Ltd. and Raymond James Ltd. (the “Canadian Underwriters”) and in the United States by Wells Fargo Securities, LLC, Raymond James (USA) Ltd. and B. Riley FBR, Inc. (the “U.S. Underwriters”, and together with the Canadian Underwriters, the “Underwriters”) pursuant to an underwriting agreement (the “Underwriting Agreement”) dated as of , 2019. B. Riley FBR, Inc. is not registered to sell securities in any Canadian jurisdiction and, accordingly, will only sell the Offered Shares outside of Canada. The Offering Price has been determined by negotiation between IMV and the Underwriters. See “Plan of Distribution”.
Price: $ per Offered Share
| Price to the Public | Underwriters’ Commission(1) | Net Proceeds to the Corporation(2) | ||||||||||
| Per Offered Share | $ | $ | $ | |||||||||
| Total(3) | $ | $ | $ | |||||||||
Notes:
| (1) | The Underwriters will receive a commission (the “Underwriters’ Commission”) equal to % of the gross proceeds of the Offering. |
| (2) | After deducting the Underwriters’ Commission but before deducting the expenses of the Offering, estimated to be $ . |
| (3) | The Corporation has also granted the Underwriters an option (the “Option”), exercisable in whole or in part in the sole discretion of the Underwriters for a period of 30 days from the closing of the Offering, to purchase up to an additional Offered Shares (the “Additional Offered Shares”) at the Offering Price. If the Option is exercised in full, the total price to the public will be $ , the total Underwriters’ Commission will be $ , and the net proceeds to the Corporation, after deducting the Underwriters’ Commission but before deducting the estimated expenses of the Offering, will be $ . |
This Prospectus Supplement qualifies the grant of the Option and the distribution of the Additional Offered Shares:
Underwriters’ Position |
Maximum Size or Number of Securities Available |
Exercise Period or Acquisition Date |
Exercise Price or Average Acquisition Price | |||
| Option | Additional Offered Shares | 30 days from the date of this Prospectus Supplement | $ per Additional Offered Share |
All references to “Offered Shares” in this Prospectus Supplement include the Additional Offered Shares and all references
to the “Offering” shall include the Option, as the context permits or requires.
Subject to applicable laws, the Underwriters may, in connection with the Offering, over-allocate, over-allot or effect transactions which stabilize or maintain the market price of the Common Shares at levels other than those which might otherwise prevail in the open market. Such transactions, if commenced, may be discontinued at any time. See “Plan of Distribution”.
A purchaser who acquires Additional Offered Shares acquires those securities under this Prospectus Supplement, regardless of whether the Underwriters exercise the Option or acquire such shares on the open market.
An investment in the Offered Shares involves a high degree of risk. Prospective investors should carefully consider the risk factors described in and/or incorporated by reference in this Prospectus Supplement and the Base Shelf Prospectus. See “Cautionary Statement Regarding Forward-Looking Statements” and “Risk Factors”.
The common shares of the Corporation (the “Common Shares”) are listed on the Toronto Stock Exchange (the “TSX”) under the symbol “IMV” and on the Nasdaq Capital Market (“NASDAQ”) under the symbol “IMV”. On February 27, 2019, the last trading day of the Common Shares on the TSX and NASDAQ before the date hereof, the closing price of the Common Shares was C$6.80 and US$4.80, respectively. An application has been made to list the Offered Shares on the TSX and NASDAQ. Listing of the Offered Shares will be subject to the Corporation fulfilling the respective listing requirements of each of the TSX and NASDAQ. Closing of the Offering is subject to usual closing conditions.
The Underwriters conditionally offer the Offered Shares on behalf of the Corporation, as principals, and, subject to prior sale, if, as and when issued by the Corporation and delivered and accepted by the Underwriters in accordance with the conditions contained in the Underwriting Agreement referred to under “Plan of Distribution” and subject to approval of certain legal matters relating to the Offering relating to Canadian law on behalf of the Corporation by McCarthy Tétrault LLP. Certain legal matters in connection with the Offering relating to U.S. law will be passed upon for the Corporation by Troutman Sanders LLP. Certain legal matters in connection with the Offering will be passed upon for the Underwriters by Blake, Cassels & Graydon LLP, with respect to Canadian Law, and by Cooley LLP, with respect to U.S. law.
| ii |
The Underwriters propose to offer the Offered Shares initially at the Offering Price. After the Underwriters have made a reasonable effort to sell all of the Offered Shares at the Offering Price, the Offering Price may be decreased and may be further changed from time to time to an amount not greater than the Offering Price, and the compensation realized by the Underwriters in respect of the Offered Shares will be decreased by the amount that the aggregate price paid by purchasers for the Offered Shares is less than the gross proceeds paid by the Underwriters to the Corporation. See “Plan of Distribution”.
Subscriptions for Offered Shares will be received subject to rejection or allotment in whole or in part and the right is reserved to close the subscription books at any time without notice. It is expected that closing of the Offering will occur on or about March , 2019 or such other date as the Corporation and the Underwriters may agree upon (the “Closing Date”).
We expect that delivery of the Offered Shares will be made to investors on or about the Closing Date, which is the third business day following the date of pricing of the Offered Shares (such settlement being referred to as “T+3”). Under Rule 15c6-1 of the Securities Exchange Act of 1934, as amended, trades in the secondary market generally are required to settle in two business days unless the parties to any such trade expressly agree otherwise. Accordingly, purchasers who wish to trade the Offered Shares on the date of pricing of the Offered Shares or the next succeeding business days will be required, by virtue of the fact that the Offered Shares initially will settle in T+3, to specify an alternate settlement cycle at the time of any such trade to prevent failed settlement and should consult their own advisers.
It is anticipated that an electronic position representing the Offered Shares will be issued, registered and deposited in electronic form with CDS Clearing and Depositary Services Inc. (“CDS”) or its nominee pursuant to the book-based system. Except in limited circumstances, no beneficial holder of Offered Shares will receive definitive certificates representing their interest in the Offered Shares. Further, except in limited circumstances, beneficial holders of Offered Shares will receive only a customer confirmation from the Underwriters or other registered dealer who is a CDS participant and from or through whom a beneficial interest in the Offered Shares is acquired.
The offering of Securities hereunder is made by a Canadian issuer that is permitted, under a multijurisdictional disclosure system (“MJDS”) adopted by the United States and Canada, to prepare this Prospectus Supplement and the Base Shelf Prospectus in accordance with Canadian disclosure requirements. Prospective investors should be aware that such requirements are different from those of the United States. Annual financial statements for the year ended December 31, 2017 included or incorporated herein have been prepared in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board (“IFRS”) and are subject to Canadian auditing and auditor independence standards and thus may not be comparable to financial statements of United States companies.
The enforcement by investors of civil liabilities under the United States federal securities laws may be affected adversely by the fact that the Corporation is incorporated or organized under the laws of a foreign country, that some or all of its officers and directors may be residents of a foreign country, that some or all of the Underwriters or experts named in this Prospectus Supplement and the Base Shelf Prospectus may by residents of a foreign country and that all or a substantial portion of the assets of the Corporation and said persons may be located outside the United States.
THESE SECURITIES HAVE NOT BEEN APPROVED OR DISAPPROVED BY THE SEC, THE SECURITIES COMMISSION OF ANY STATE OF THE UNITED STATES OR ANY CANADIAN SECURITIES REGULATOR NOR HAVE ANY OF THE FOREGOING PASSED UPON THE ACCURACY OR ADEQUACY OF THIS PROSPECTUS SUPPLEMENT AND THE BASE SHELF PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
| iii |
Prospective investors should be aware that the acquisition, holding or disposition of the Offered Shares described herein may have tax consequences both in the United States and in Canada. Such consequences for investors who are resident in, or citizens of, the United States and Canada may not be described fully herein. You should read the tax discussion contained in this Prospectus Supplement and consult your own tax advisor with respect to your own particular circumstances. See the sections titled “Certain Canadian Federal Income Tax Considerations”, “Certain U.S. Federal Income Tax Considerations” and “Risk Factors”.
The Offered Shares may only be sold in those jurisdictions where offers and sales are permitted. This Prospectus Supplement is not an offer to sell or a solicitation of an offer to buy the Offered Shares in any jurisdiction in which it is unlawful. Prospective investors should be aware that the acquisition or disposition of the Offered Shares described in this Prospectus Supplement may have tax consequences in Canada or elsewhere, depending on each particular existing or prospective investor’s specific circumstances.
Julia Gregory, Wayne Pisano, Albert Scardino and Markus Warmuth, members of the board of directors of the Corporation, all reside outside of Canada and have appointed IMV Inc., 130 Eileen Stubbs Avenue, Suite 19, Dartmouth, Nova Scotia, Canada, B3B 2C4, as agent for service of process. Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process.
On May 2, 2018, the Corporation filed articles of amendment to give effect to a consolidation of its Common Shares on the basis of 1 post-consolidation Common Share for each 3.2 pre-consolidation Common Shares. The post-consolidation Common Shares began trading on TSX on May 10, 2018. Historical trading prices and volumes have been amended to reflect the 3.2 for 1 consolidation.
The Corporation’s head office and registered office is located at 130 Eileen Stubbs Avenue, Suite 19, Dartmouth, Nova Scotia, Canada, B3B 2C4.
| Wells Fargo Securities | Raymond James |
B. Riley FBR
Prospectus dated , 2019
| iv |
TABLE OF CONTENTS
Preliminary Prospectus Supplement
TABLE OF CONTENTS
Base Shelf Prospectus
| ii |
This document is in two parts. The first part is this Prospectus Supplement, which describes the terms of the Offering and adds to and updates information in the accompanying Base Shelf Prospectus and the documents incorporated by reference therein. The second part is the accompanying Base Shelf Prospectus, which gives more general information, some of which may not apply to the Offering. This Prospectus Supplement is deemed to be incorporated by reference into the accompanying Base Shelf Prospectus solely for the purposes of this Offering. This Prospectus Supplement may add, update or change information contained in the accompanying Base Shelf Prospectus. Before investing, you should carefully read both this Prospectus Supplement and the accompanying Base Shelf Prospectus together with the additional information about the Corporation to which you are referred in the sections of this Prospectus Supplement and the Base Shelf Prospectus entitled “Documents Incorporated by Reference”.
Purchasers of Offered Shares should rely only on the information contained in or incorporated by reference into this Prospectus Supplement and the Base Shelf Prospectus. The Corporation has not authorized anyone to provide purchasers with different or additional information. If information in this Prospectus Supplement is inconsistent with the Base Shelf Prospectus or the information incorporated by reference, you should rely on this Prospectus Supplement. If anyone provides purchasers with different or additional information, purchasers should not rely on it. Neither the Corporation nor the Underwriters are making an offer to sell or seeking an offer to buy the Offered Shares in any jurisdiction where the offer or sale is not permitted. Purchasers should assume that the information contained in this Prospectus Supplement and the Base Shelf Prospectus is accurate only as of the date on the front of those documents and that information contained in any document incorporated by reference is accurate only as of the date of that document, regardless of the time of delivery of this Prospectus Supplement and the Base Shelf Prospectus or of any sale of the Offered Shares. The Corporation’s business, financial condition, results of operations and prospects may have changed since those dates.
This Prospectus Supplement and the Base Shelf Prospectus include references to trade names and trademarks of other companies, which trade names and trademarks are the properties of their respective owners.
The corporate website of the Corporation is www.imv-inc.com. The information on the Corporation’s website is not intended to be included or incorporated by reference into this Prospectus Supplement and the Base Shelf Prospectus and prospective purchasers should not rely on such information when deciding whether or not to invest in the Offered Shares.
Statistical information and other data relating to the pharmaceutical and biotechnology industry included in this Prospectus Supplement and the Base Shelf Prospectus are derived from recognized industry reports published by industry analysts, industry associations and/or independent consulting and data compilation organizations. Market data and industry forecasts used throughout this Prospectus Supplement and the Base Shelf Prospectus were obtained from various publicly available sources. Although the Corporation believes that these independent sources are generally reliable, the accuracy and completeness of the information from such sources are not guaranteed and have not been independently verified.
This Prospectus Supplement and the Base Shelf Prospectus are part of the U.S. Registration Statement. This Prospectus Supplement and the Base Shelf Prospectus do not contain all of the information set forth in the U.S. Registration Statement, certain parts of which are omitted in accordance with the rules and regulations of the SEC, or the schedules or exhibits that are part of the U.S. Registration Statement. Investors in the United States should refer to the U.S. Registration Statement and the exhibits thereto for further information with respect to IMV and the Offered Shares.
In this Prospectus Supplement, the Base Shelf Prospectus and the documents incorporated by reference herein and therein, unless the context otherwise requires, references to “IMV” or the “Corporation” refer to IMV Inc., together with its subsidiary, Immunovaccine Technologies Inc.
| S-3 |
The consolidated financial statements incorporated by reference into this Prospectus Supplement and the Base Shelf Prospectus and the other documents incorporated by reference into this Prospectus Supplement and the Base Shelf Prospectus, and the financial data derived from those consolidated financial statements included in this Prospectus Supplement and the Base Shelf Prospectus, are presented in Canadian dollars, unless otherwise specified, and have been prepared in accordance with IFRS. References in this Prospectus Supplement to “dollars”, “C$” or “$” are to Canadian dollars. United States dollars are indicated by the symbol “US$”.
The following table lists, for each period presented, the high and low exchange rates, the average of the exchange rates during the period indicated, and the exchange rates at the end of the period indicated, for one Canadian dollar, expressed in United States dollars, based on the exchange rate published by the Bank of Canada for the applicable periods. Periods prior to March 1, 2017 are based on the noon rate published by the Bank of Canada. Periods from and after March 1, 2017 are based on the closing exchange rate published by the Bank of Canada.
| Year ended December 31, | Nine months ended September 30, | |||||||||||||||||||
| 2018 | 2017 | 2016 | 2018 | 2017 | ||||||||||||||||
| High for the period | 0.8138 | 0.8245 | 0.7972 | 0.8138 | 0.8245 | |||||||||||||||
| Low for the period | 0.7330 | 0.7276 | 0.6854 | 0.7513 | 0.7276 | |||||||||||||||
| End of period | 0.7330 | 0.7971 | 0.7448 | 0.7725 | 0.8013 | |||||||||||||||
| Average for the period | 0.7721 | 0.7708 | 0.7548 | 0.7769 | 0.7657 | |||||||||||||||
On February 27, 2019, the closing exchange rate for one Canadian dollar, expressed in United States dollars, as reported by the Bank of Canada, was C$1.00 = US$0.7607.
Cautionary Statement Regarding Forward-Looking Statements
Certain statements contained in this Prospectus Supplement, the Base Shelf Prospectus and the documents incorporated by reference in this Prospectus Supplement and the Base Shelf Prospectus may constitute “forward-looking information” within the meaning of applicable securities laws in Canada and “forward-looking statements” within the meaning of the United States Private Securities Legislation Reform Act of 1995 (collectively, “forward-looking statements”) which involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements of the Corporation, or industry results, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. When used in this Prospectus Supplement, such statements reflect current expectations regarding future events and operating performance and speak only as of the date of this Prospectus Supplement. Forward-looking statements may use such words as “will”, “may”, “could”, “intends”, “potential”, “plans”, “believes”, “expects”, “projects”, “estimates”, “anticipates”, “continue”, “predicts” or “should” and other similar terminology.
Forward-looking statements include, but are not limited to, statements relating to:
| - | the Corporation’s business strategy; |
| - | statements with respect to the sufficiency of the Corporation’s financial resources to support its activities; |
| - | potential sources of funding; |
| - | the Corporation’s ability to obtain necessary funding on favorable terms or at all; |
| - | the Corporation’s expected expenditures and accumulated deficit level; |
| - | the Corporation’s expected outcomes from its ongoing and future research and research collaborations; |
| - | the Corporation’s ability to obtain necessary regulatory approvals; |
| - | the Corporation’s expected outcomes from its pre-clinical studies and trials; |
| S-4 |
| - | the Corporation’s exploration of opportunities to maximize shareholder value as part of the ordinary course of its business through collaborations, strategic partnerships and other transactions with third parties; |
| - | the Corporation’s plans for the research and development of certain product candidates; |
| - | the Corporation’s strategy for protecting its intellectual property; |
| - | the Corporation’s ability to identify licensable products or research suitable for licensing and commercialization; |
| - | the Corporation’s ability to obtain licences on commercially reasonable terms; |
| - | the Corporation’s plans for generating revenue; |
| - | the Corporation’s plans for future clinical trials; |
| - | the Corporation’s expected use of the net proceeds from this offering; and |
| - | the Corporation’s hiring and retention of skilled staff. |
The forward-looking statements reflect the Corporation’s current views with respect to future events, are subject to risks and uncertainties, and are based upon a number of estimates and assumptions that, while considered reasonable by the Corporation, are inherently subject to significant business, economic, competitive, political and social uncertainties and contingencies. Many factors could cause the Corporation’s actual results, performance or achievements to be materially different from any future results, performance, or achievements that may be expressed or implied by such forward-looking statements, including, among others:
| - | obtaining additional funding on reasonable terms when necessary; |
| - | positive results of pre-clinical studies and clinical trials; |
| - | the Corporation’s ability to successfully develop existing and new products; |
| - | the Corporation’s ability to hire and retain skilled staff; |
| - | the products and technology offered by the Corporation’s competitors; |
| - | general business and economic conditions; |
| - | the Corporation’s ability to protect its intellectual property; |
| - | the Corporation’s ability to manufacture its products and to meet demand; |
| - | the general regulatory environment in which the Corporation operates; and |
| - | obtaining necessary regulatory approvals and the timing in respect thereof. |
Should one or more of these risks or uncertainties materialize, or should the assumptions set out in the section entitled “Risk Factors” underlying those forward-looking statements prove incorrect, actual results may vary materially from those described herein. These forward-looking statements are made as of the date of this Prospectus Supplement or the date of the Base Shelf Prospectus or, in the case of documents incorporated by reference in this Prospectus Supplement or the Base Shelf Prospectus, as of the date of such documents, and the Corporation does not intend, and does not assume any obligation, to update these forward-looking statements, except as required by law. There is no assurance that such statements will prove to be accurate as actual results and future events could differ materially from those anticipated in such statements. Purchasers are cautioned that forward-looking statements are not guarantees of future performance and accordingly purchasers are cautioned not to put undue reliance on forward-looking statements due to the inherent uncertainty therein. New factors emerge from time to time, and it is not possible for management of the Corporation to predict all of these factors or to assess in advance the impact of each such factor on the Corporation’s business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statement.
| S-5 |
The forward-looking statements contained in this Prospectus Supplement and the Base Shelf Prospectus are expressly qualified by the foregoing cautionary statements and are made as of the date of this Prospectus Supplement or the Base Shelf Prospectus. The Corporation does not undertake any obligation to publicly update or revise any forward-looking statements, except as required by applicable securities laws. Purchasers should read this entire Prospectus Supplement and the Base Shelf Prospectus and consult their own professional advisors to assess the income tax, legal, risk factors and other aspects of their investment in the Offered Shares.
DOCUMENTS INCORPORATED BY REFERENCE
This Prospectus Supplement is deemed to be incorporated by reference into the Base Shelf Prospectus solely for the purpose of the Offering. Other information has also been incorporated by reference in the Base Shelf Prospectus from documents filed with the securities commissions or similar authorities in Canada. Copies of the documents incorporated herein by reference may be obtained on request without charge from the Corporate Secretary of the Corporation at 130 Eileen Stubbs Avenue, Suite 19, Dartmouth, Nova Scotia, Canada, B3B 2C4 (telephone (902) 492-1819), and are also available electronically at www.sedar.com.
In addition to the continuous disclosure obligations of the Corporation under the securities laws of certain provinces of Canada, the Corporation is subject to certain of the information requirements of the U.S. Securities Exchange Act of 1934, as amended (the “Exchange Act”), and in accordance therewith file reports and other information with the SEC. Under MJDS, some reports and other information may be prepared in accordance with the disclosure requirements of Canada, which requirements are different from those of the United States. As a foreign private issuer, the Corporation is exempt from the rules under the Exchange Act prescribing the furnishing and content of proxy statements, and the Corporation’s officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, the Corporation may not be required to publish financial statements as promptly as U.S. companies. A free copy of any public document filed by IMV with the SEC’s Electronic Data Gathering and Retrieval (EDGAR) system is available from the SEC’s website at www.sec.gov.
The following documents filed with the securities commissions or similar authorities in the provinces of British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Québec, Nova Scotia and Newfoundland and Labrador are specifically incorporated by reference in and form an integral part of this Prospectus Supplement:
| (i) | the annual information form of the Corporation dated March 20, 2018 for the year ended December 31, 2017 (the “AIF”); |
| (ii) | the audited annual consolidated financial statements of the Corporation and the notes thereto for the years ended December 31, 2017 and 2016, together with the auditor’s report thereon; |
| (iii) | the management’s report on financial position and operating results of the Corporation for the year ended December 31, 2017 (the “Annual MD&A”), except for the “Letter to Shareholders” which is specifically excluded and is not incorporated by reference herein; |
| (iv) | the unaudited interim condensed consolidated financial statements of the Corporation and the notes thereto for the nine months ended September 30, 2018 and 2017; |
| (v) | the management’s report on financial position and operating results of the Corporation for the three and nine-month period ended September 30, 2018, except for the “Letter to Shareholders” which is specifically excluded and is not incorporated by reference herein; |
| (vi) | the management information circular dated March 29, 2018 relating to the annual and special meeting of shareholders of the Corporation held on May 1, 2018; |
| (vii) | the material change report dated February 2, 2018 relating to a bought-deal financing agreement to sell Common Shares (the “February 2018 Public Offering”); |
| S-6 |
| (viii) | the material change report dated February 21, 2018 relating to the closing of the February 2018 Public Offering; and |
| (ix) | the material change report dated May 10, 2018 relating the NASDAQ listing, the consolidation of the Common Shares and the name change. |
Any documents of the Corporation of the type referred to in the preceding paragraph and any material change reports (excluding any confidential material change reports) filed by the Corporation with a securities commission or similar regulatory authority in Canada on or after the date of this Prospectus Supplement and prior to the termination of the Offering shall be deemed to be incorporated by reference into this Prospectus Supplement and the Base Shelf Prospectus.
In addition, to the extent that any document or information incorporated by reference into this Prospectus Supplement and the Base Shelf Prospectus is included in any report on Form 6-K, Form 40-F or Form 20-F (or any respective successor form) that is filed with or furnished to the SEC by the Corporation after the date of this Prospectus, such document or information shall be deemed to be incorporated by reference as an exhibit to the U.S. Registration Statement of which this Prospectus forms a part. In addition, the Corporation may incorporate by reference into this Prospectus, or the U.S. Registration Statement of which it forms a part, other information from documents that the Corporation will file with or furnish to the SEC pursuant to Section 13(a) or 15(d) of the Exchange Act, if and to the extent expressly provided therein.
Any statement contained in this Prospectus Supplement, the Base Shelf Prospectus or in a document incorporated or deemed to be incorporated by reference in this Prospectus Supplement or the Base Shelf Prospectus shall be deemed to be modified or superseded for purposes of this Prospectus Supplement to the extent that a statement contained herein or in any other subsequently filed document which also is or is deemed to be incorporated by reference in this Prospectus Supplement or the Base Shelf Prospectus modifies or supersedes such statement. The modifying or superseding statement need not state that it has modified or superseded a prior statement or include any other information set forth in the document that it modifies or supersedes. Any statement so modified or superseded shall not be deemed to constitute a part of this Prospectus Supplement or the Base Shelf Prospectus, except as so modified or superseded.
You should rely only on the information contained in or incorporated by reference in this Prospectus Supplement and the Base Shelf Prospectus and on the other information included in the U.S. Registration Statement of which the Base Shelf Prospectus forms a part. The Corporation is not making an offer of Offered Shares in any jurisdiction where the offer is not permitted by law.
DOCUMENTS FILED AS PART OF THE U.S. REGISTRATION STATEMENT
The following documents have been or will be (through post-effective amendment or incorporation by reference) filed with the SEC as part of the U.S. Registration Statement of which this Prospectus is a part insofar as required by the SEC’s Form F-10:
| · | the documents listed under “Documents Incorporated by Reference” in this Prospectus;. |
| · | the form of Underwriting Agreement described in this Prospectus Supplement; |
| · | the consent of PricewaterhouseCoopers LLP, the Corporation’s independent auditor; |
| · | the consent of McCarthy Tétrault LLP, the Corporation’s Canadian counsel; and |
| · | powers of attorney of the Corporation’s directors and officers, as applicable. |
Marketing Materials and Contractual Right
Before filing this Prospectus Supplement in respect of this Offering, the Corporation and the Underwriters intend to hold road shows that potential investors in the United States and certain provinces in Canada will be able to attend. The Corporation and the Underwriters may provide marketing materials to those potential investors in connection with those road shows.
| S-7 |
In doing so, the Corporation and the Underwriters are relying on a provision in applicable Canadian securities legislation that allows issuers in certain U.S. cross-border offerings to not have to file marketing materials relating to those road shows on SEDAR at www.sedar.com or include or incorporate by reference those marketing materials in the Base Shelf Prospectus. To rely on this exemption, the Corporation and the Underwriters must give contractual rights to Canadian investors in the event the marketing materials contain a misrepresentation.
Accordingly, the Corporation and the Underwriters signing the certificate contained in this Prospectus Supplement have agreed that in the event the marketing materials relating to the road shows described above contain a misrepresentation (as defined in securities legislation in certain provinces of Canada), a purchaser resident in a province of Canada who was provided with those marketing materials in connection with the road shows and who purchases Common Shares under the Prospectus Supplement during the period of distribution shall have, without regard to whether the purchaser relied on the misrepresentation, rights against the Corporation and each Underwriter signing the certificate contained in the Prospectus Supplement with respect to the misrepresentation which are equivalent to the rights under the securities legislation of the jurisdiction of Canada where the purchaser is resident, subject to the defences, limitations and other terms of that legislation, as if the misrepresentation was contained in the Prospectus Supplement.
However, this contractual right does not apply (i) to the extent that the contents of the marketing materials relating to the road shows have been modified or superseded by a statement in the Prospectus Supplement, and (ii) to any “comparables” (as such term is defined in National Instrument 44-102 – Shelf Distributions) in the marketing materials provided in accordance with applicable securities legislation.
The following description of IMV is derived from selected information about the Corporation contained in the documents incorporated by reference and does not contain all of the information about the Corporation and its business that should be considered before investing in the securities. This Prospectus Supplement, the accompanying Base Shelf Prospectus and the documents incorporated by reference herein and therein should be reviewed and considered by prospective purchasers in connection with their investment in the Offered Shares. This Prospectus Supplement may add to, update or change information in the accompanying Base Shelf Prospectus. You should carefully read this entire Prospectus Supplement and the accompanying Base Shelf Prospectus, including the risks and uncertainties discussed in the section titled “Risk Factors,” and the information incorporated by reference in this Prospectus Supplement, including the consolidated financial statements of the Corporation, before making an investment decision. If you invest in the Corporation’s securities, you are assuming a high degree of risk.
The Corporation was incorporated on May 18, 2007 under the name of Rhino Resources Inc. pursuant to the Canada Business Corporations Act. On September 28, 2009, the Corporation changed its name to Immunovaccine Inc. and consolidated its outstanding share capital on a 5 to 1 basis. On May 2, 2018, the Corporation changed its name to IMV Inc. and consolidated its outstanding share capital on a 3.2 to 1 basis.
The Corporation has one wholly-owned subsidiary, Immunovaccine Technologies Inc. (“IVT”), which is incorporated under the laws of Nova Scotia.
The Corporation’s head and registered office is located at 130 Eileen Stubbs Avenue, Suite 19, Dartmouth, Nova Scotia, Canada, B3B 2C4.
| S-8 |
| Common Shares Offered by the Corporation | Common Shares | |
| Common Shares to be Outstanding After This Offering | Common Shares (or Common Shares if the Option is exercised in full). | |
| Use of Proceeds |
The Corporation estimates that the net proceeds from the Offering will be approximately $ million (or $ million if the Option is exercised in full) after deducting the Underwriters’ Commission of $ (or $ if the Option is exercised in full) and Offering expenses, which are estimated to be $ .
The Corporation expects to use the net proceeds of the Offering to accelerate the development of DPX-Survivac in combination with Keytruda as part of the basket trial select advanced or recurrent solid tumours in bladder, liver (hepatocellular carcinoma), ovarian or non-small-cell lung (NSCLC) cancers, as well as tumours shown to be positive for the microsatellite instability high (MSI-H) biomarker and for general corporate purposes. See the section titled “Use of Proceeds” on page S-19 of this Prospectus Supplement. | |
| Risk Factors | Investing in the Common Shares involves a high degree of risk. Please read the information contained in and incorporated by reference under the section titled “Risk Factors” beginning on page S-29 of this Prospectus Supplement, and under similar headings in the other documents that are filed after the date hereof and incorporated by reference into this Prospectus Supplement. | |
| NASDAQ Symbol | “IMV” | |
| TSX Symbol | “IMV” |
The number of Common Shares that will be outstanding immediately after this Offering as shown above is based on 44,981,427 Common Shares outstanding as of September 30, 2018. The number of Common Shares outstanding as of September 30, 2018 as used throughout this Prospectus Supplement, unless otherwise indicated, excludes:
| - | 1,448,478 Common Shares issuable upon exercise of outstanding options outstanding as of September 30, 2018, with a weighted average exercise price of $4.05; |
| - | an aggregate of 1,461,181 Common Shares reserved for future issuance under our stock option plans as of September 30, 2018. |
Subsequent to September 30, 2018, and through the date of this Prospectus Supplement:
| - | we granted an additional 273,100 stock options with a weighted average exercise price of $7.22 per share; and |
| - | we issued an additional 119,336 Common Shares upon the exercise of warrants, and 86,704 Common Shares upon the exercise of stock options. |
See the section titled “Prior Sales” on page S-25 of this prospectus supplement.
Unless otherwise indicated, all information in this Prospectus Supplement assumes no exercise by the Underwriters of the Option and no exercise of outstanding options or warrants.
| S-9 |
Overview
IMV is a clinical-stage biopharmaceutical company dedicated to making immunotherapy more effective, more broadly applicable, and more widely available to people facing cancer and other serious diseases. IMV is headquartered in Halifax, Nova Scotia and currently has 54 employees. IMV is pioneering a new class of immunotherapies based on the Corporation’s proprietary drug delivery platform (“DPX”). This patented technology, leverages a novel mechanism of action (“MOA”) discovered by the Corporation. This MOA does not release the active ingredients at the site of injection but forces an active uptake and delivery of active ingredients into immune cells and lymph nodes. It enables the programming of immune cells in vivo, which are aimed at generating powerful new synthetic therapeutic capabilities. DPX’s no release MOA can be leveraged to generate “first-in-class” T cell therapies with the potential to be transformative in the treatment of cancer.
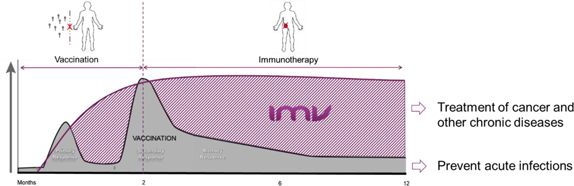
Figure 1: Illustrative representation of IMV’s DPX new MOA
DPX also has multiple manufacturing advantages; it is fully synthetic, can accommodate hydrophilic and hydrophobic compounds, is amenable to a wide-range of applications (for example, peptides, small molecules, RNA/DNA or antibodies), and provides long-term stability as well as low cost of goods.
| S-10 |
The Corporation’s first cancer immunotherapy uses survivin-based peptides licensed from Merck KGaA, on a world-wide exclusive basis, formulated in DPX (“DPX-Survivac”). DPX-Survivac leverages the MOA of DPX to generate a constant flow of T cells in the blood that are targeted against survivin expressed on cancer cells. It is comprised of five minimal MHC class I peptides to activate naïve T cells against survivin.
Survivin is a well characterized and recognized tumor associated antigen known to be expressed during fetal development and across most tumour cell types, but is rarely present in normal, non-malignant adult cells survivin controls key cancer processes (apoptosis, cell division and metastasis) and has been associated with chemotherapy resistance and cancer progression. It has been shown that survivin was expressed in all 60 different human tumour lines used in the National Cancer Institute’s cancer drug-screening program and documented in the literature to be overexpressed in more than 20 indications. |
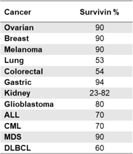
Figure 2: Examples of % of patients with survivin expression in different indications |
The Corporation’s clinical strategy is to establish monotherapy activity in order to increase value and de-risk clinical development and to target late-stage unmet medical needs for a shorter path to clinical demonstration and regulatory approval.
The Corporation is focussing on fast path to market in ovarian and diffuse large B cell lymphoma (“DLBCL”) cancers and on repeating its clinical demonstrations of activity in other indications.
The Corporation has completed Phase 1 and 1b clinical trials in ovarian cancer in a maintenance setting after first or second line in 56 subjects.
The results provided first in human clinical demonstration of DPX-Survivac mechanism of action (survivin-specific T cell flow in the blood) and potential as treatment. 87% of patients generated one of highest T cell level reported in the literature and that level was maintained during a year with repeated injection every two months. One patient with measurable residual disease experienced a -46% tumor reduction.
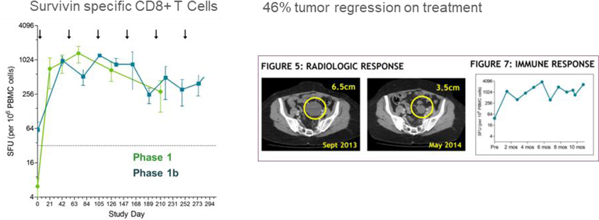
Figure 3: Phase 1/1b result (ASCO 2015)
DPX-Survivac is currently being tested in:
| · | a phase 2 clinical trial which evaluates DPX -Survivac in an open label safety and efficacy study in ovarian cancer patients with advanced platinum-sensitive and resistant ovarian cancer with sum of base line target lesions per Recist criteria less than five centimeters; |
| S-11 |
| · | two investigator-sponsored phase 2 clinical trials in combination with checkpoint inhibitor Keytruda® (pembrolizumab) of Merck & Co Inc. (“Merck”) in patients with recurrent, platinum-resistant and sensitive ovarian cancer and in patients with measurable or recurrent DLBCL. In these cases, the primary and secondary endpoints include objective response rate (“ORR”), tumor regression, toxicity profile and duration of responses; and |
| · | a phase 2 basket trial in combination with Merck’s Keytruda® (pembrolizumab), in five different indications in patients with select advanced or recurrent solid tumours in bladder, liver (hepatocellular carcinoma), ovarian or non-small-cell lung (NSCLC) cancers, as well as tumours shown to be positive for the microsatellite instability high (MSI-H) biomarker. |
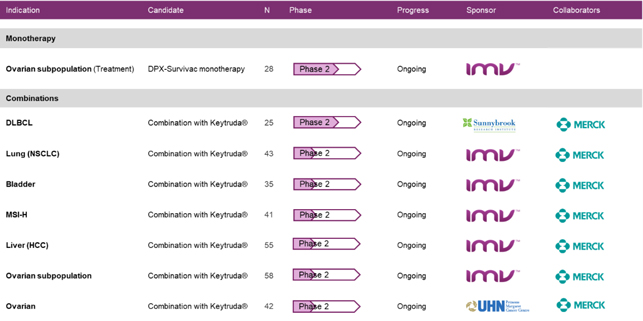
Figure 4: Ongoing phase 2 clinical trials
In infectious disease vaccine applications, the Corporation has completed a demonstration phase 1 clinical trial with a target against the respiratory syncytial virus (RSV). The Corporation also has a commercial licensing agreement with Zoetis for the development of two cattle vaccines and is also conducting several research and clinical collaborations, including a collaboration with the Dana-Farber Cancer Institute for Human Papillomavirus (HPV) related cancers and with Leidos, Inc. in the United States for the development of vaccine candidates for malaria and the Zika virus.
For further information, see “Description of the Business” in the AIF.
Appointment of a New Board Member
On November 6, 2018, the Corporation announced that it has appointed seasoned biopharmaceutical executive Markus Warmuth, MD, to its Board of Directors. Dr. Warmuth currently serves as an Entrepreneur in Residence at the life science venture capital firm Third Rock Ventures. He brings more than 20 years of drug discovery experience and scientific acumen, with a strong focus on developing targeted therapy and immune-oncology programs, to his new role on IMV’s Board.
Phase 1b/2 Clinical Trial
On November 20, 2018, the Corporation announced an amendment to its phase 1b/2 clinical trial evaluating the safety and efficacy of IMV’s lead candidate, DPX-Survivac, in combination with either 100 mg or 300 mg of epacadostat in patients with recurrent ovarian cancer.
| S-12 |
Review of new data from the phase 1b portion of the clinical trial demonstrates a high response rate and a durable clinical benefit in a subpopulation of patients with a clinical marker predictive of a response to DPX-Survivac and correlated to its novel MOA. New data includes:
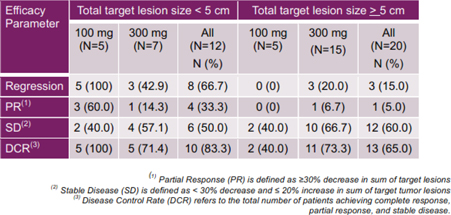
| · | Efficacy signals in the subpopulation of patients who received 100 mg dose epacadostat (n=5) included 100% tumor regressions and 100% disease control rate; and 60% of these patients (3/5) reached a best response of a partial response (“PR”); |
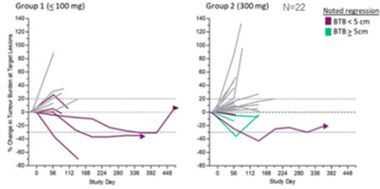
Figure 5: Phase 1b Tumor Regressions (ESMO-IO 2018)
| · | Long duration of clinical benefits observed in responders that lasted beyond treatment duration (1 year) for a median duration of 590 days, including one patient who has passed the two-year mark without disease progression and prolonged tumor control observed in 3 out 4 PR in that subpopulation. |
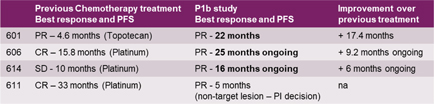
Figure 6: Longer progression-free Survival (PFS) than previous chemotherapy treatment (ESMO IO 2018)
| · | Clinical benefit correlated to DPX-Survivac’s MOA and clinical study primary endpoints: survivin-specific T cells in the blood and T cell infiltration into tumors; and, |
| · | The safety profile of DPX-Survivac is consistent with the profile observed in the Corporation’s previously reported studies. |
| S-13 |
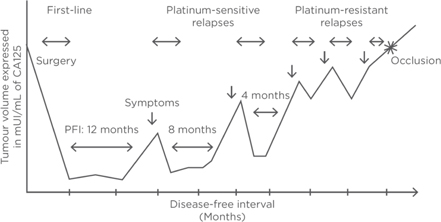
Figure 7: Ovarian cancer disease-free interval (Source: Ovarian cancer, Giornelli, EMJ 2017)
Based on 300 mg cohort results, IMV and Incyte Corporation (“Incyte”) have agreed to stop dosing patients with epacadostat. IMV will continue the phase 1b/2 trial as a monotherapy study evaluating DPX-Survivac in the recurrent ovarian cancer subpopulation. IMV will inform and work with investigators to appropriately modify the study in a manner consistent with the best interests of each patient.
IMV and Incyte will continue to explore the potential of additional combination studies.
On December 13, 2018, the Corporation announced that investigators shared new positive data from the Corporation’s ongoing DeCidE1 (DPX-Survivac with low dose CyclophosphamIDe and Epacadostat) clinical trial at the 2018 ESMO Immuno-Oncology Congress. The phase 1b/2 study is evaluating the safety and efficacy of the combination of IMV’s lead candidate DPX-Survivac, low dose cyclophosphamide, and 100 mg or 300mg of Incyte’s IDO1 enzyme inhibitor epacadostat in patients with advanced recurrent ovarian cancer.
Key findings include:
| · | Evidence of a clinical marker based on Baseline Tumor Burden (“BTB”), a measure of tumor size predictive of patient response to DPX-Survivac |
| o | 37.5% (12/32) of evaluable study subjects began treatment with a non-bulky disease defined as BTB < 5 cm. |
| o | 73% (8/11) of tumor regressions and 80% of clinical responses (4/5) observed in a subset of patients with BTB < 5 cm. |
| · | Responders showing prolonged duration of clinical benefits reaching up to more than two years, surpassing the progression-free interval from their previous chemotherapy treatment |
| · | Robust systemic survivin-specific T cell responses and evidence of survivin-specific T cells tumor infiltration correlated with clinical benefits |
| o | 100% of durable clinical responses correlated with T cell infiltration, |
| · | Epacadostat triggered inhibition of the conversion of tryptophan into kynurenine that was dose dependent |
| · | Cohort demographics were balanced and the combination yielded a tolerable safety profile |
| S-14 |
At the time of data cut-off, 53 patients were enrolled in the phase 1b clinical trial, including 14 from the 100 mg epacadostat dosing cohort and 39 from 300 mg epacadostat cohort. Based on 300 mg cohort results, IMV and Incyte agreed to stop dosing patients with epacadostat before completion of the study. Patients who completed at least one CT scan, as required per the trial protocol, were evaluable for response analysis.
71% of patients were evaluable for responses in the 100 mg cohort and 56% in the 300mg dose cohort. At time of data cut-off, 8 participants remained on treatment and were being evaluated for clinical responses.
New Role of Senior Director, Investor Relations and Communications
On December 5, 2018, the Corporation announced that Marc Jasmin has joined the Corporation in the newly created role of Senior Director, Investor Relations and Communications.
First Patient Dosed in Phase 1 Clinical Trial Evaluating Neoepitopes Formulated in IMV’s DPX Delivery Platform in Ovarian Cancer Patients
On January 17, 2019, the Corporation announced that the first patient has been treated in the phase 1 trial evaluating neoepitopes formulated in the Corporation’s proprietary DPX delivery platform in patients with ovarian cancer. The study is part of the Corporation’s DPX-NEO program, which is an ongoing collaboration between UConn Health and IMV to develop neoepitope-based anti-cancer therapies.
Investigators will assess the safety and efficacy of using patient-specific neoepitopes discovered at UConn Health and formulated in IMV’s proprietary DPX-based delivery technology in women with ovarian cancer. Investigators plan to enroll up to 15 patients in the phase 1 study. UConn Health is funding the trial with IMV providing materials and counsel.
Epitopes are the part of the biological molecule that is the target of an immune response. Neoepitopes are the mutated proteins produced by a patient's own tumors. Neoepitope immunotherapies target these patient-specific proteins and have been referred to as ‘the next immunotherapy frontier’.
Clinical update for DPX-Survivac program in ovarian cancer following positive feedback from U.S. FDA
On January 30, 2019, the Corporation announced an update on its clinical program for its lead investigational treatment, DPX-Survivac, as a potential monotherapy in advanced recurrent ovarian cancer. In December 2018, IMV met with the U.S. Food and Drug Administration (“FDA”) in a Type B meeting to discuss the results to date of its Decide1 clinical trial and continuing development plan, as well as to obtain agency guidance on a potential accelerated regulatory pathway for DPX-Survivac as a T-cell immunotherapy for the treatment of advanced ovarian cancer in patients with progressing disease.
| S-15 |
FDA meeting highlights
The purpose of IMV’s Type B meeting with the FDA was to request feedback on the design of the clinical program for DPX-Survivac. This program includes the continuing Decide phase 2 clinical study and a potential future registration trial for accelerated approval in a subset of ovarian cancer patients.
The FDA reviewed the Corporation’s proposed clinical development plan and acknowledged the potential for accelerated approvals in advanced ovarian cancer based on ORR according to Recist 1.1 criteria with reported median duration of response (“DOR”). In addition, the FDA provided important guidance on clinical design considerations for different lines of therapy and platinum-sensitive and resistant patient populations.
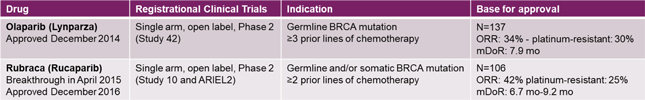
Figure 8: Examples of previous US FDA accelerated approvals in ovarian cancer (source: FDA website)
In addition, IMV submitted a protocol amendment for a predictive enrichment approach to the phase 2 Decide trial, and further discussed those details with the FDA during the Type B meeting. The phase 2 primary end point, based on ORR per Recist 1.1 criteria, is intended to confirm the high response rate and duration of clinical benefits observed in previously announced results in a patient population defined by a clinical biomarker based on BTB.
The Corporation believes there is still an urgent medical need in advanced recurrent ovarian cancer (Sources: 1. NCCN Guidelines Ovarian Cancer V2.2018;.SEER Ovarian Cancer; JCO, vol 33; 32 Nov 2015, Gyn Onc 133(2014) 624-631):
| · | Nearly 70% of ovarian cancers are diagnosed in advanced stage |
| · | The overall 5-year survival rate is 46.5%, and only 29% for advanced disease |
| · | Most patients develop advanced, platinum-resistant, poor prognosis disease |
| · | Limited options with current single-agents 6-30% response rates and mPFS of 2.1-4.2 months |
The Corporation believes that it has the potential to be “best-in-class” in the competitive landscape of recurrent ovarian cancer as other immunotherapeutic treatments tested in this patient population (Incyte’s epacadostat, Merck’s Keytruda, and Pfizer/Merck KGaA’s Bavencio) are unlikely to proceed into registration trials based on published results available:
| S-16 |
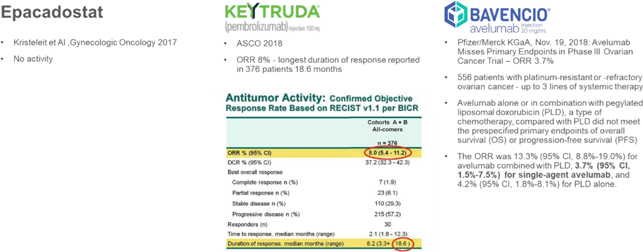
Figure 9: Recurrent ovarian cancer immunotherapy competitive landscape
Multiple clinical sites are now open for enrolment in the DECIDE phase 2 trial. Subject to phase 2 results, IMV plans to schedule a follow-up meeting with the FDA to finalize the design of a potential pivotal trial based on ORR and DOR.
The Corporation has completed key clinical milestones in the last quarter of 2018 and is expecting a significant number of additional clinical results in 2019 and 2020:
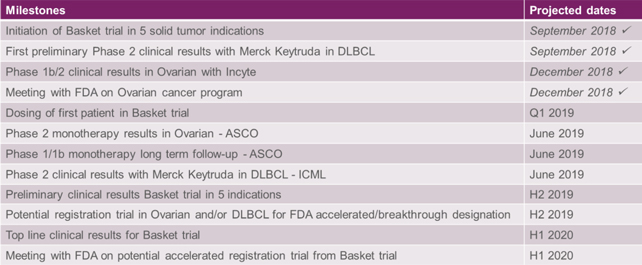
Figure 10: Key clinical milestones completed in Q4 2018 and expected in 2019-2020
Expected Fiscal Year 2018 Earnings Results
While we have not yet finalized our full financial results for the fiscal year ended December 31, 2018, we expect to report that we had approximately $14,917,000 in cash and cash equivalents as of December 31, 2018. This amount is preliminary, has not been audited and is subject to change upon completion of our audited financial statements for the year ended December 31, 2018. Additional information and disclosures would be required for a more complete understanding of our financial position and results of operations as of December 31, 2018.
| S-17 |
Since September 30, 2018, the end of the most recent interim reporting period of the Corporation, there have been no material changes in the loan capital of the Corporation and no material changes in the share capital of the Corporation on a consolidated basis other than as outlined under “Prior Sales.” For information on the exercise of options pursuant to the stock option plan of the Corporation and other outstanding convertible securities, see the section titled “Prior Sales.”
The following table summarizes the Corporation’s share and loan capital as at September 30, 2018 on an actual basis and as adjusted to give effect to the issue and sale of Common Shares in this Offering at a public offering price of $ per Common Share and after deducting the Underwriters’ Commission and estimated Offering expenses. This table should be read in conjunction with the audited consolidated annual financial statements of the Corporation as at and for the years ended December 31, 2017 and 2016. Figures are in thousands of Canadian dollars, except share data.
| As at September 30, 2018 (Unaudited) | ||||||||
| Actual | As Adjusted(1) | |||||||
| Cash and cash equivalents | $ | 20,271,000 | $ | |||||
| Long-Term Debt | $ | 7,493,000 | $ | |||||
| Equity: | ||||||||
| Common Shares | 44,981,427 | (2) | ||||||
| Common Share Purchase Warrants | 297,372 | 297,372 | ||||||
| Stock Options | 1,448,478 | 1,448,478 | ||||||
| Deferred share units | 224,368 | 224,368 | ||||||
| Total Equity | ||||||||
| Total Share Capital (fully diluted) | 46,951,645 | |||||||
| (1) | Based on the issuance of Offered Shares for aggregate gross proceeds of $ , less the Underwriters’ Commission of $ and expenses of the Offering, estimated at $ . |
| (2) | Up to Common Shares if the Option is exercised in full for aggregate gross proceeds of up to $ , less the Underwriters’ Commission of $ and expenses of the Offering, estimated at $ . |
| S-18 |
The estimated net proceeds to be received by the Corporation under the Offering will be approximately $ (up to approximately $ if the Option is exercised in full), after deducting the Underwriters’ Commission and the estimated expenses in connection with this Offering of approximately $ .
The Corporation has negative operating cash flow and it is expected that the proceeds from the Offering will be used to fund operating cash flow. The Corporation expects its current cash and cash equivalents, together with the anticipated net proceeds from this Offering (not including any exercise of the Option), to fund its planned operations until the fourth quarter of 2020. Specifically, the Corporation intends to use approximately $ million of the net proceeds from the Offering to accelerate the development of DPX-Survivac in combination with Keytruda as part of the basket trial select advanced or recurrent solid tumours in bladder, liver (hepatocellular carcinoma), ovarian or non-small-cell lung (NSCLC) cancers, as well as tumours shown to be positive for the microsatellite instability high (MSI-H) biomarker; and the remainder for general corporate purposes.
The key business objective the Corporations intends to meet with the net proceeds is to accelerate the development of DPX-Survivac, its lead product candidate in combination with other Keytruda in survivin-expressing tumors, for select advanced or recurrent solid tumours in bladder, liver (hepatocellular carcinoma), ovarian or non-small-cell lung (NSCLC) cancers, as well as tumours shown to be positive for the microsatellite instability high (MSI-H) biomarker. This objective will require additional capital exceeding the cash on hand resources of the Corporation even after giving effect to the Offering and the exercise, if any, of the Option. In addition, actual costs and development time may exceed management’s current expectations. It is unlikely that the Corporation will generate sufficient operating cash flow to meet the total capital obligation in the proposed development time frame. Accordingly, the Corporation will need to raise additional capital in the future over and above the proceeds from the current Offering.
If the Option is exercised in whole or in part, the Corporation expects to use the additional net proceeds from such exercise to support its key business objectives and for general corporate purposes.
While the Corporation intends to spend the net proceeds of the Offering as stated above, there may be circumstances where, for sound business reasons, a re-allocation of funds may be necessary or advisable. The actual amount that the Corporation spends in connection with each of the intended uses of proceeds may vary significantly from the amounts specified above, and will depend on a number of factors, including those listed under the heading “Risk Factors” in this Prospectus Supplement and the accompanying Base Shelf Prospectus and the documents incorporated by reference herein and therein.
Negative Cash Flow
The Corporation has incurred significant operating losses and negative cash flows from operations since inception and has an accumulated deficit of $85,073,000 as of September 30, 2018. The ability of the Corporation to continue as a going concern is dependent upon raising additional financing through equity and non-dilutive funding and partnerships. There can be no assurance that the Corporation will have sufficient capital to fund its ongoing operations, develop or commercialize any products without future financings. These material uncertainties cast significant doubt as to the Corporation’s ability to meet its obligations as they come due and, accordingly, the appropriateness of the use of accounting principles applicable to a going concern. If the Corporation is unable to obtain additional financing when required, the Corporation may have to substantially reduce or eliminate planned expenditures or the Corporation may be unable to continue operations.
The Corporation’s ability to continue as a going concern is dependent upon its ability to fund its research and development programs and defend its patent rights. It is expected that proceeds from the Offering will be used to fund anticipated negative cash flow from operating activities, as described above.
| S-19 |
Pursuant to the Underwriting Agreement dated , 2019 among the Corporation and Wells Fargo Securities Canada, Ltd. and Raymond James Ltd., as the representatives (the “Representatives”) of the Underwriters named below and the joint book-running managers of the Offering, the Corporation has agreed to sell to the Underwriters, and each of the Underwriters has severally (and not jointly, nor jointly and severally) agreed to purchase, as principals, or cause to be purchased, on the Closing Date, the respective number of Offered Shares shown opposite its name below, at the Offering Price for aggregate gross proceeds of $ payable in cash to the Corporation against delivery of the Offered Shares, subject to compliance with all necessary legal requirements and the terms and conditions of the Underwriting Agreement. The Offering Price was determined by arm’s length negotiations between the Corporation and the Underwriters, with reference to the prevailing market price of the Common Shares.
| Underwriter | Number of Shares | |||
| Wells Fargo Securities Canada, Ltd. | ||||
| Raymond James Ltd. | ||||
| B. Riley FBR, Inc. | ||||
| Total | ||||
The Underwriters are offering the Offered Shares, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel, including the validity of the Offered Shares, and other conditions contained in the underwriting agreement, such as the receipt by the underwriters of officers’ certificates and legal opinions. The obligations of the Underwriters under the Underwriting Agreement are several and not joint (nor joint and several) and may be terminated at their discretion upon the occurrence of certain stated events. Such events include, but are not limited to (i) the occurrence of any material adverse change or any development that could reasonably be expected to result in a material adverse change, in the condition (financial or other), results of operations, business, properties, management or prospects of the Corporation and its subsidiary taken as a whole, whether or not arising in the ordinary course of business that is so material and adverse as to make it impractical or inadvisable to proceed with the offering of the Securities, (ii) the occurrence of any material adverse change in the financial markets in the United States, Canada or the international financial markets, any declaration of a national emergency or war by the United States or Canada, any outbreak of hostilities or escalation thereof or other calamity or crisis or any change or development involving a prospective change in national or international political, financial or economic conditions (including, without limitation, as a result of terrorist activities), in each case the effect of which is such as to make it, in the judgment of the Representatives, impracticable or inadvisable to market the Offered Shares or to enforce contracts for the sale of the Offered Shares, (iii) (A) trading in any securities of the Corporation has been suspended or materially limited on Nasdaq or TSX by such exchange, the SEC, any qualifying authority or any other governmental authority having jurisdiction, or (B) trading generally on the Nasdaq Stock Market or the TSX has been suspended or limited, or minimum or maximum prices for trading have been fixed, or maximum ranges for prices have been required, by any of said exchanges or by order of the SEC, FINRA or any other governmental authority, or (C) the occurrence of a material disruption in commercial banking or securities settlement or clearance services in the United States or Canada, or (iv) declaration of a banking moratorium by either United States federal, Canadian federal or New York authorities. The Underwriters are, however, obligated, to take up and pay for (or cause the payment for) all of the Offered Shares if any of the Offered Shares are purchased under the Underwriting Agreement. The Underwriters may offer selling group participation to other registered dealers, with compensation to be negotiated between the Underwriters and such selling group participants, but at no additional cost to the Corporation. Pursuant to the terms of the Underwriting Agreement, the Corporation has agreed to pay certain expenses incurred by the Underwriters in connection with the Offering. The Corporation has also agreed pursuant to the terms of the Underwriting Agreement to indemnify the Underwriters, their affiliates and their respective directors, employees, shareholders and agents against certain liabilities, including liabilities under the U.S. Securities Act, and expenses and to contribute to payments that the Underwriters may be required to make in respect thereof.
Commissions and Discounts
In consideration for the services provided by the Underwriters in connection with the Offering and pursuant to the terms of the Underwriting Agreement, the Corporation has agreed to pay the Underwriters the Underwriters’ Commission, equal to % of the aggregate gross proceeds of the Offering (including in respect of any Additional Offered Shares sold upon exercise of the Option). The compensation realized by the Underwriters will be decreased by the amount that the aggregate price paid by the purchasers for the Offered Shares is less than the gross proceeds paid by the Underwriters to the Corporation. Any such reduction will not affect the proceeds received by the Corporation.
The Underwriters propose to offer the Offered Shares to the public initially at the Offering Price and to dealers at that price less a concession not in excess of $ per share.. Without affecting the firm obligation of the Underwriters to purchase the Offered Shares in accordance with the Underwriting Agreement, the Underwriters may decrease the Offering Price of the Offered Shares which they sell under this Prospectus Supplement after they have made a reasonable effort to sell all such Offered Shares at the Offering Price. The sale by the Underwriters of Offered Shares at a price of less than the Offering Price will have the effect of reducing the compensation realized by the Underwriters by the amount that the aggregate price paid by the purchasers for Offered Shares is less than the gross proceeds paid by the Underwriters for the Offered Shares.
The following table shows the public Offering Price, underwriting discounts and commissions and proceeds before expenses to the Corporation. The information assumes either no exercise or full exercise by the Underwriters of the Option.
| Per Share | Without Option | With Option | ||||||||||
| Public offering price | $ | $ | $ | |||||||||
| Underwriting discounts and commissions | $ | $ | $ | |||||||||
| Proceeds, before expenses, to the Corporation | $ | $ | $ | |||||||||
It is estimated that expenses payable by the Corporation in connection with this Offering, other than the underwriting discounts and commissions referred to above, will be approximately $ . The Corporation has also agreed to reimburse the Underwriters for up to $75,000 for certain FINRA-related and other expenses incurred in connection with this Offering. In accordance with FINRA Rule 5110, this reimbursement fee is deemed underwriting compensation for this Offering.
| S-20 |
Option to Purchase Additional Shares
The Corporation has also granted the Underwriters the Option, exercisable in whole or in part in the sole discretion of the Underwriters for a period of 30 days from the Closing Date, to purchase, from time to time, up to an additional Additional Offered Shares at the Offering Price, less underwriting discounts and commissions. If the Underwriters exercise the Option, each Underwriter will be obligated, subject to specified conditions, to purchase a number of additional shares proportionate to that Underwriter’s initial purchase commitment as indicated in the table above.
Price Stabilization, Short Positions and Penalty Bids
In connection with the Offering, the Underwriters may purchase and sell Common Shares in the open market. These transactions may include short sales, purchases on the open market to cover positions created by short sales and stabilizing transactions. Short sales involve the sale by the Underwriters of a greater number of shares than they are required to purchase in the offering. “Covered” short sales are sales made in an amount not greater than the Option described above. The Underwriters may close out any covered short position by either exercising the Option or purchasing shares in the open market. In determining the source of Common Shares to close out the covered short position, the Underwriters will consider, among other things, the price of Common Shares available for purchase in the open market as compared to the price at which they may purchase Common Shares through the Option. “Naked” short sales are sales in excess of the Option. The Underwriters must close out any naked short position by purchasing Common Shares in the open market. A naked short position is more likely to be created if the Underwriters are concerned that there may be downward pressure on the price of the Common Shares in the open market after pricing that could adversely affect investors who purchase in the Offering. Stabilizing transactions consist of various bids for or purchases of Common Shares made by the Underwriters in the open market prior to the closing of the Offering.
The Underwriters may also impose a penalty bid. This occurs when a particular Underwriter repays to the Underwriters a portion of the underwriting discount received by it because the Representatives have repurchased shares sold by or for the account of such Underwriter in stabilizing or short covering transactions.
Similar to other purchase transactions, the Underwriters’ purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of the Common Shares or preventing or retarding a decline in the market price of the Common Shares. As a result, the price of the Common Shares may be higher than the price that might otherwise exist in the open market. The Underwriters may conduct these transactions on NASDAQ, the TSX, in the over-the-counter market or otherwise.
Neither the Corporation nor any of the Underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of the Common Shares. In addition, neither the Corporation nor any of the Underwriters make any representation that the Representatives will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
Electronic Distribution
In connection with the Offering, certain of the Underwriters or securities dealers may distribute this Prospectus Supplement and the Base Shelf Prospectus electronically.
No Sale of Similar Securities
The Corporation and its executive officers and directors will not, subject to certain limited exceptions, directly or indirectly, offer, issue, sell or grant any Common Shares or securities convertible into, exchangeable for, or otherwise exercisable to acquire Common Shares or other equity securities of the Corporation for a period of 90 days after the Closing Date, without the prior written consent of Wells Fargo Securities Canada Ltd and Raymond James Ltd., on behalf of the Underwriters, such consent not to be unreasonably withheld.
| S-21 |
Settlement
Subscriptions for Offered Shares will be received subject to rejection or allotment in whole or in part and the right is reserved to close the subscription books at any time without notice. It is anticipated that an electronic position representing the Offered Shares will be issued, registered and deposited in electronic form with CDS or its nominee pursuant to the book-based system. Except in limited circumstances, no beneficial holder of Offered Shares will receive definitive certificates representing their interest in the Offered Shares. Further, except in limited circumstances, beneficial holders of Offered Shares will receive only a customer confirmation from the Underwriters or other registered dealer who is a CDS participant and from or through whom a beneficial interest in the Offered Shares is acquired.
We expect that delivery of the Offered Shares will be made to investors on or about the Closing Date, which is the third business day following the date of pricing of the Offered Shares (such settlement being referred to as “T+3”). Under Rule 15c6-1 of the Securities Exchange Act of 1934, as amended, trades in the secondary market generally are required to settle in two business days unless the parties to any such trade expressly agree otherwise. Accordingly, purchasers who wish to trade the Offered Shares on the date of pricing of the Offered Shares or the next succeeding business days will be required, by virtue of the fact that the Offered Shares initially will settle in T+3, to specify an alternate settlement cycle at the time of any such trade to prevent failed settlement and should consult their own advisers.
NASDAQ and Toronto Stock Exchange Listing
The Common Shares are listed on the TSX under the symbol “IMV” and on NASDAQ under the symbol “IMV.” An application has been made to list the Offered Shares on the TSX and NASDAQ. Listing of the Offered Shares will be subject to the Corporation fulfilling the listing requirements of the TSX and NASDAQ. Closing of the Offering is subject to usual closing conditions.
Other Relationships
The Underwriters and certain of their affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. Some of the Underwriters and certain of their affiliates may in the future engage in investment banking and other commercial dealings in the ordinary course of business with the Corporation and its affiliates, for which they may in the future receive customary fees, commissions and expenses.
In addition, in the ordinary course of their business activities, the Underwriters and their affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers.
Sales Inside the United States and Canada
The Offering is being made concurrently in the United States and in the provinces of British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Québec, Nova Scotia and Newfoundland and Labrador. The Offered Shares will be offered in the United States through certain of the Underwriters, either directly or indirectly, through their respective U.S. broker-dealer affiliates or agents. The Offered Shares will be offered in certain provinces of Canada through those Underwriters or their affiliates who are registered to offer the Offered Shares for sale in such provinces and such other registered dealers as may be designated by the Underwriters. Subject to applicable law and the provisions of the Underwriting Agreement, the Underwriters may offer the Offered Shares outside of the United States and Canada.
| S-22 |
The Offering is being made in Canada and in the United States pursuant to the MJDS implemented by the securities regulatory authorities in the United States and Canada. The Offered Shares will be offered in the United States and Canada by the Underwriters either directly or through their U.S. or Canadian broker-dealer affiliates or agents, as applicable. Subject to applicable law, the Underwriters may offer the Offered Shares outside of Canada and the United States.
Sales Outside the United States and Canada
No action has been taken in any jurisdiction (except the United States and Canada) that would permit a public offering of the Common Shares, or the possession, circulation or distribution of this Prospectus Supplement, the accompanying Base Shelf Prospectus or any other material relating to the Corporation or the Common Shares in any jurisdiction where action for that purpose is required. Accordingly, the Offered Shares may not be offered or sold, directly or indirectly, and none of this Prospectus Supplement, the accompanying Base Shelf Prospectus or any other offering material or advertisements in connection with the offering of common shares may be distributed or published, in or from any country or jurisdiction except in compliance with any applicable rules and regulations of any such country or jurisdiction.
Each of the Underwriters may arrange to sell the Offered Shares in certain jurisdictions outside the United States and Canada, either directly or through affiliates, where they are permitted to do so. In that regard, Wells Fargo Securities, LLC may arrange to sell Common Shares in certain jurisdictions through an affiliate, Wells Fargo Securities International Limited (“WFSIL”). WFSIL is a wholly-owned indirect subsidiary of Wells Fargo & Company and an affiliate of Wells Fargo Securities, LLC. WFSIL is a U.K. incorporated investment firm regulated by the Financial Conduct Authority. Wells Fargo Securities is the trade name for certain corporate and investment banking services of Wells Fargo & Company and its affiliates, including Wells Fargo Securities, LLC and WFSIL.
European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive, or each, a Relevant Member State, with effect from and including the date on which the Prospectus Directive is implemented in that Relevant Member State, or the Relevant Implementation Date, an offer of Common Shares described in this Prospectus Supplement and accompanying Base Shelf Prospectus may not be made to the public in that Relevant Member State except that, with effect from and including the Relevant Implementation Date, an offer to the public in that Relevant Member State of any such Common Shares may be made at any time under the following exemptions under the Prospectus Directive:
| (a) | to any legal entity which is a qualified investor as defined in the Prospectus Directive; |
| (b) | to fewer than 150 natural or legal persons (other than qualified investors as defined in the Prospectus Directive), subject to obtaining the prior consent of the Underwriters for any such offer; or |
| (c) | in any other circumstances falling within Article 3(2) of the Prospectus Directive, |
provided that no such offer of Common Shares referred to in (a) to (c) above shall require the Corporation or any Underwriter to publish a prospectus pursuant to Article 3 of the Prospective Directive or supplement a prospectus pursuant to Article 16 of the Prospectus Directive.
Each purchaser of Common Shares described in this Prospectus Supplement and accompanying Base Shelf Prospectus located within a Relevant Member State will be deemed to have represented, acknowledged and agreed that it is a “qualified investor” within the meaning of Article 2(1)(e) of the Prospectus Directive.
For the purpose of this Prospectus Supplement and accompanying Base Shelf Prospectus, the expression an “offer to the public” in relation to any Common Shares in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the Common Shares to be offered so as to enable an investor to decide to purchase or subscribe for the Common Shares, as the expression may be varied in the Relevant Member State, and the expression “Prospectus Directive” means Directive 2003/71/EC, and includes any relevant implementing measure in the Relevant Member State.
| S-23 |
United Kingdom
This Prospectus Supplement and accompanying Base Shelf Prospectus are only being distributed to, and are only directed at, persons in the United Kingdom that are qualified investors within the meaning of Article 2(1)(e) of the Prospective Directive that are also (i) “investment professionals” falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended, or the Order, (ii) high net worth entities falling within Article 49(2)(a) to (d) of the Order or (iii) persons to whom it may otherwise lawfully be communicated (all such persons together being referred to as relevant persons). This Prospectus Supplement and accompanying Base Shelf Prospectus are directed only at relevant persons and must not be acted on or relied on in the United Kingdom by persons who are not relevant persons. Any investment or investment activity to which this Prospectus Supplement and accompanying Base Shelf Prospectus relates is available only to relevant persons, and will be engaged in only with relevant persons.
Each Underwriter has represented and agreed that:
| · | it has only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement to engage in investment activity (within the meaning of Section 21 of the Financial Services and Markets Act 2000, as amended, or the FSMA) received by it in connection with the issue or sale of the common shares in circumstances in which Section 21(1) of the FSMA does not apply to the Corporation; and |
| · | it has complied and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to the common shares in, from or otherwise involving the United Kingdom. |
Switzerland
This document as well as any other material relating to the Common Shares that are the subject of the Offering contemplated by this Prospectus Supplement and accompanying Base Shelf Prospectus do not constitute an issue prospectus pursuant to Article 652a or Article 1156 of the Swiss Code of Obligations. The Common Shares will not be listed on the SWX Swiss Exchange and, therefore, the documents relating to the Common Shares, including, but not limited to, this document, do not claim to comply with the disclosure standards of the listing rules of SWX Swiss Exchange and corresponding prospectus schemes annexed to the listing rules of the SWX Swiss Exchange.
The Common Shares are being offered in Switzerland by way of a private placement, i.e. to a small number of selected investors only, without any public offer and only to investors who do not purchase the Common Shares with the intention to distribute them to the public. The investors will be individually approached by the Corporation from time to time.
This document as well as any other material relating to the Common Shares is personal and confidential and does not constitute an offer to any other person. This document may only be used by those investors to whom it has been handed out in connection with the offering described herein and may neither directly nor indirectly be distributed or made available to other persons without the express consent of the Corporation. It may not be used in connection with any other offer and shall in particular not be copied and/or distributed to the public in (or from) Switzerland.
Investors in China
This Prospectus Supplement does not constitute a public offer of Common Shares, whether by sale or subscription, in the People's Republic of China (the “PRC”). The Common Shares are not being offered or sold directly or indirectly in the PRC to or for the benefit of, legal or natural persons of the PRC.
Further, no legal or natural persons of the PRC may directly or indirectly purchase any of the Common Shares without obtaining all prior PRC’s governmental approvals that are required, whether statutorily or otherwise. Persons who come into possession of this document are required by the issuer and its representatives to observe these restrictions.
| S-24 |
DESCRIPTION OF securities being distributed
IMV’s authorized share capital consists of an unlimited number of Common Shares and preferred shares (the “Preferred Shares”) issuable in series, all without par value. As of September 30, 2018, a total of 44,981,427 Common Shares and no Preferred Shares were issued and outstanding. 1,448,478 Common Shares were issuable upon the exercise of outstanding stock options, 297,372 Common Shares were issuable upon the exercise of outstanding warrants and 224,368 Common Shares were issuable upon the conversion of outstanding deferred share units (“DSUs”).
On May 2, 2018, the Corporation filed articles of amendment to give effect to a consolidation of its Common Shares on the basis of 1 post-consolidation Common Share for each 3.2 pre-consolidation Common Shares. All current and historical per share data and number of Common Shares issued and outstanding is presented on a post-consolidation basis, unless otherwise noted.
The Common Shares of the Corporation rank junior to the Preferred Shares with respect to the payment of dividends, return of capital and distribution of assets in the event of liquidation, dissolution or winding-up of the Corporation. Subject to the prior rights of the holders of Preferred Shares, the holders of Common Shares are entitled to receive dividends as and when declared by the Board of Directors of the Corporation. In the event of liquidation, dissolution or winding-up of the Corporation, subject to the prior rights of the holders of Preferred Shares, the holders of Common Shares are entitled to receive all the remaining property and assets of the Corporation. The holders of Common Shares are entitled to receive notice of and to attend and to vote at all meetings of the shareholders of the Corporation and each Common Share, when represented at any meeting of the shareholders of the Corporation, carries the right to one vote.
The Corporation has not paid any dividends to date on the Common Shares. The Corporation does not currently expect to pay any dividends on the Common Shares for the foreseeable future.
The following table sets out the details of the issuance by the Corporation of Common Shares, options to purchase Common Shares, warrants to purchase Common Shares, DSUs, if any, during the 12-month period before the date of this Prospectus Supplement:
| Security | Number(1) | Price(1) | Issuance Date | |||||||
| Stock Options(2) | 390,625 | C$6.40 | March 21, 2018 | |||||||
| Common Shares(3) | 625 | C$3.20 | March 22, 2018 | |||||||
| Common Shares(3) | 3,025 | C$0.90 | March 22, 2018 | |||||||
| Common Shares(3) | 773 | N/A (4) | March 26, 2018 | |||||||
| Common Shares(3) | 3,125 | C$3.20 | March 27, 2018 | |||||||
| Deferred share units(5) | 15,675 | C$6.24 | March 31, 2018 | |||||||
| Stock options(6) | 4,688 | C$6.40 | April 1, 2018 | |||||||
| Common Shares(3) | 5,084 | N/A (7) | April 3, 2018 | |||||||
| Common Shares(3) | 1,702 | N/A (8) | April 4, 2018 | |||||||
| Common Shares(3) | 1,972 | N/A (9) | April 5, 2018 | |||||||
| Common Shares(3) | 10,144 | C$0.90 | April 11, 2018 | |||||||
| Common Shares(10) | 22,891 | C$2.30 | April 19, 2018 | |||||||
| Common Shares(10) | 17,469 | C$2.30 | April 23, 2018 | |||||||
| Common Shares(10) | 15,625 | C$2.30 | April 24, 2018 | |||||||
| Common Shares(3) | 1,721 | N/A (11) | April 27, 2018 | |||||||
| Common Shares(3) | 10,938 | C$0.90 | April 30, 2018 | |||||||
| Common Shares(12) | 15,625 | C$2.53 | May 1, 2018 | |||||||
| Common Shares(10) | 2,344 | C$2.30 | May 1, 2018 | |||||||
| S-25 |
| Security | Number(1) | Price(1) | Issuance Date | |||||||
| Common Shares(10) | 4,250 | C$2.30 | May 3, 2018 | |||||||
| Common Shares(3) | 12,839 | N/A (13) | May 15, 2018 | |||||||
| Common Shares(10) | 62,500 | C$2.30 | May 17, 2018 | |||||||
| Common Shares(10) | 7,812 | C$2.30 | May 18, 2018 | |||||||
| Common Shares(10) | 18,467 | C$2.30 | May 23, 2018 | |||||||
| Common Shares(3) | 15,625 | C$2.53 | May 23, 2018 | |||||||
| Common Shares(3) | 15,625 | C$2.11 | May 23, 2018 | |||||||
| Common Shares(3) | 3,125 | C$3.20 | May 23, 2018 | |||||||
| Common Shares(3) | 15,625 | C$2.37 | May 23, 2018 | |||||||
| Common Shares(3) | 15,625 | C$2.11 | May 23, 2018 | |||||||
| Common Shares(10) | 10,812 | C$2.30 | May 24, 2018 | |||||||
| Common Shares(10) | 4,250 | C$2.30 | May 25, 2018 | |||||||
| Common Shares(10) | 4,109 | C$2.30 | May 28, 2018 | |||||||
| Common Shares(10) | 31,250 | C$2.30 | May 30, 2018 | |||||||
| Common Shares(10) | 16,328 | C$2.30 | May 31, 2018 | |||||||
| Common Shares(10) | 6,250 | C$2.30 | June 1, 2018 | |||||||
| Common Shares(10) | 184,437 | C$2.30 | June 5, 2018 | |||||||
| Deferred share units(14) | 10,226 | US$6.42 | June 7, 2018 | |||||||
| Common Shares(10) | 1,500,567 | C$2.30 | June 8, 2018 | |||||||
| Deferred share units(15) | 16,958 | C$6.66 | June 30, 2018 | |||||||
| Common Shares(3) | 4,970 | N/A (16) | July 19, 2018 | |||||||
| Common Shares(3) | 14,500 | C$2.37 | August 30, 2018 | |||||||
| Common Shares(3) | 9,708 | C$2.40 | August 30, 2018 | |||||||
| Common Shares(3) | 31,250 | C$2.37 | August 30, 2018 | |||||||
| Common Shares(3) | 31,250 | C$2.11 | August 30, 2018 | |||||||
| Common Shares(3) | 1,375 | C$3.20 | August 30, 2018 | |||||||
| Deferred share units(17) | 21,230 | US$5.77 | September 30, 2018 | |||||||
| Common Shares(12) | 18,375 | C$2.53 | October 3, 2018 | |||||||
| Deferred share units(18) | 11,665 | US$5.63 | November 6, 2018 | |||||||
| Common Shares(3) | 16,874 | N/A (19) | November 9, 2018 | |||||||
| Common Shares(3) | 1,624 | N/A (20) | November 20, 2018 | |||||||
| Stock options(21) | 30,000 | C$7.39 | November 26, 2018 | |||||||
| Common Shares(22) | 86,538 | C$4.22 | December 5, 2018 | |||||||
| Common Shares(3) | 1,563 | C$2.37 | December 17, 2018 | |||||||
| Deferred share units(23) | 21,318 | US$5.03 | December 31, 2018 | |||||||
| Common Shares(3) | 21,192 | N/A (24) | January 2, 2019 | |||||||
| Common Shares(3) | 11,636 | N/A (25) | January 7, 2019 | |||||||
| Common Shares(3) | 7,316 | N/A (26) | January 11, 2019 | |||||||
| Common Shares(3) | 15,625 | C$2.37 | January 11, 2019 | |||||||
| Common Shares(3) | 9,022 | N/A (27) | January 15, 2019 | |||||||
| Stock options(28) | 243,100 | C$7.20 | January 15, 2019 | |||||||
| Common Shares(22) | 14,423 | C$4.22 | January 22, 2019 | |||||||
| Common Shares(3) | 446 | N/A (29) | January 31, 2019 | |||||||
| Common Shares(3) | 1,406 | C$2.37 | February 15, 2019 | |||||||
| Common Shares(3) | 1,938 | C$2.40 | February 27, 2019 | |||||||
| (1) | On May 2, 2018, the Corporation filed articles of amendment to give effect to a consolidation of its Common Shares on the basis of 1 post-consolidation Common Share for each 3.2 pre-consolidation Common Shares. Historical information with regards to issuance of Common Shares, options to purchase Common Shares, warrants to purchase Common Shares and DSUs has been amended to reflect the 3.2 to 1 consolidation. Fractions have been rounded up or down to the nearest whole number and prices have been rounded up or down to the nearest cent. |
| (2) | Grant of stock options governed by the Corporation’s stock option plan exercisable at a price of C$6.40 per Common Share until March 21, 2023. |
| (3) | Common Shares issued upon exercise of stock options. |
| (4) | Cashless exercise of 1,563 options. |
| (5) | DSUs issued pursuant to the Corporation’s deferred share unit plan (the “DSU Plan”) at a deemed price of C$6.24 per DSU. Each DSU entitles the holder thereof to receive one Common Share on the terms and conditions set forth in the DSU Plan. |
| S-26 |
| (6) | Grant of stock options governed by the Corporation’s stock option plan exercisable at a price of $6.40 per Common Share until April 1, 2023. |
| (7) | Cashless exercise of 7,813 options. |
| (8) | Cashless exercise of 1,992 options. |
| (9) | Cashless exercise of 2,306 options. |
| (10) | Common Shares issued upon exercise of common share purchase warrants (the “2016 Warrants”) being part of units issued pursuant to a bought-deal private placement of units. Each 2016 Warrant entitles its holder to purchase one Common Share at a price of C$2.30 per Common Share until June 8, 2018. |
| (11) | Cashless exercise of 2,020 options. |
| (12) | Common shares issued upon exercise of compensation options exercisable at a price of C$2.528 per Common Share until December 9, 2018 issued as consideration to the underwriters in connection with a bought-deal private placement of Common Shares in December 2016 (the “December 2016 Private Placement”). |
| (13) | Common shares issued upon redemption of 26,051 DSUs in accordance with the terms of the DSU Plan. |
| (14) | DSUs issued pursuant to the DSU Plan at a deemed price of US$6.42 per DSU. Each DSU entitles the holder thereof to receive one Common Share on the terms and conditions set forth in the DSU Plan. |
| (15) | DSUs issued pursuant to the DSU Plan at a deemed price of C$6.66 per DSU. Each DSU entitles the holder thereof to receive one Common Share on the terms and conditions set forth in the DSU Plan. |
| (16) | Cashless exercise of 8,001 options. |
| (17) | DSUs issued pursuant to the DSU Plan at a deemed price of US$5.77 per DSU. Each DSU entitles the holder thereof to receive one Common Share on the terms and conditions set forth in the DSU Plan. |
| (18) | DSUs issued pursuant to the DSU Plan at a deemed price of US$5.63 per DSU. Each DSU entitles the holder thereof to receive one Common Share on the terms and conditions set forth in the DSU Plan. |
| (19) | Common shares issued upon redemption of 33,747 DSUs in accordance with the terms of the DSU Plan. |
| (20) | Cashless exercise of 2,438 options. |
| (21) | Grant of stock options governed by the Corporation’s stock option plan exercisable at a price of C$7.39 per Common Share until November 26, 2023. |
| (22) | Common shares issued upon exercise of compensation options exercisable at a price of C$4.224 per Common Share until June 21, 2019 issued as consideration to the underwriters in connection with a bought-deal private placement of Common Shares in June 2017. |
| (23) | DSUs issued pursuant to the DSU Plan at a deemed price of US$5.03 per DSU. Each DSU entitles the holder thereof to receive one Common Share on the terms and conditions set forth in the DSU Plan. |
| (24) | Cashless exercise of 31,250 options. |
| (25) | Cashless exercise of 17,750 options. |
| (26) | Cashless exercise of 10,157 options. |
| (27) | Cashless exercise of 13,282 options. |
| (28) | Grant of stock options governed by the Corporation’s stock option plan exercisable at a price of C$7.20 per Common Share until January 15, 2024. The grant of 93,750 of these options is subject to ratification by the shareholders of the issuer at the next meeting of shareholders. In the event the grant is not ratified, the options will automatically be cancelled. |
| (29) | Cashless exercise of 781 options. |
| S-27 |
The Common Shares are currently listed on the TSX under the symbol “IMV” and NASDAQ under the symbol “IMV”.
The following table provides the price ranges and trading volume of the Common Shares on the TSX for the periods indicated below:
| Price Ranges(1) | ||||||||||||
| High | Low | |||||||||||
| (C$) | (C$) | Total Cumulative Volume(1) | ||||||||||
| February 2018 | C$6.98 | C$5.82 | 589,178 | |||||||||
| March 2018 | C$6.66 | C$5.92 | 361,870 | |||||||||
| April 2018 | C$7.10 | C$5.18 | 551,818 | |||||||||
| May 2018 | C$9.25 | C$6.11 | 1,439,532 | |||||||||
| June 2018 | C$9.49 | C$6.29 | 1,216,081 | |||||||||
| July 2018 | C$6.75 | C$5.93 | 642,241 | |||||||||
| August 2018 | C$7.60 | C$6.10 | 385,334 | |||||||||
| September 2018 | C$7.63 | C$6.63 | 502,252 | |||||||||
| October 2018 | C$8.22 | C$6.66 | 1,120,784 | |||||||||
| November 2018 | C$8.45 | C$6.81 | 863,903 | |||||||||
| December 2018 | C$8.49 | C$6.46 | 906,574 | |||||||||
| January 2019 | C$7.75 | C$6.79 | 491,743 | |||||||||
| February 1-27, 2019 | C$7.44 | C$6.30 | 520,159 | |||||||||
| (1) | On May 2, 2018, the Corporation filed articles of amendment to give effect to a consolidation of its Common Shares on the basis of 1 post-consolidation Common Share for each 3.2 pre-consolidation Common Shares. The post-consolidation Common Shares began trading on the TSX on May 10, 2018. Historical trading prices and volumes have been amended to reflect the 3.2 for 1 consolidation. Fractions have been rounded up or down to the nearest whole number and prices have been rounded up or down to the nearest cent. |
On February 27, 2019, the last trading day of the Common Shares on the TSX before the date of this Prospectus Supplement, the closing price of the Common Shares was C$6.80.
The Common Shares began trading on NASDAQ on June 1, 2018. The following table provides the price ranges and trading volume of the Common Shares on NASDAQ for the periods indicated below:
| Price Ranges | ||||||||||||
| High | Low | |||||||||||
| (US$) | (US$) | Total Cumulative Volume | ||||||||||
| June 2018 | US$7.21 | US$4.80 | 259,366 | |||||||||
| July 2018 | US$5.15 | US$4.50 | 128,051 | |||||||||
| August 2018 | US$5.85 | US$4.71 | 79,087 | |||||||||
| September 2018 | US$5.94 | US$5.16 | 61,475 | |||||||||
| October 2018 | US$6.00 | US$5.06 | 102,506 | |||||||||
| November 2018 | US$6.31 | US$5.16 | 187,590 | |||||||||
| December 2018 | US$7.07 | US$4.71 | 274,355 | |||||||||
| January 2019 | US$5.85 | US$5.00 | 32,003 | |||||||||
| February 1-27, 2019 | US$5.67 | US$4.80 | 157,237 | |||||||||
On February 27, 2019, the last trading day of the Common Shares on NASDAQ before the date of this Prospectus Supplement, the closing price of the Common Shares was US$4.80.
| S-28 |
An investment in the Corporation’s securities involves risk. Before you invest in the Offered Shares, you should carefully consider the risks contained in or incorporated by reference into this Prospectus Supplement and the Base Shelf Prospectus, including the risks described below and in the AIF and Annual MD&A, which are incorporated by reference into this Prospectus Supplement and the Base Shelf Prospectus. The discussion of risks related to the business of the Corporation contained in or incorporated by reference into this Prospectus Supplement and the Base Shelf Prospectus comprises material risks of which the Corporation is aware. If any of the events or developments described actually occurs, the business, financial condition or results of operations of the Corporation would likely be adversely affected.
Risks Relating to the Corporation
The Corporation has incurred significant losses since inception and expects to incur losses for the foreseeable future and may never achieve or maintain profitability.
Since inception, the Corporation has incurred significant operating losses. The Corporation incurred a net loss of $12.0 million for the year ended December 31, 2017, $8.9 million for the year ended December 31, 2016 and $8.8 million for the year ended December 31, 2015. As of September 30, 2018, the Corporation had an accumulated deficit of $85,073,000. To date, the Corporation has financed operations primarily through public offerings in Canada, private placements of securities, grants and license and collaboration agreements. The Corporation has devoted substantially all efforts to research and development, including clinical trials. IMV expects to continue to incur significant expenses and increasing operating losses for at least the next several years. The Corporation anticipates that its expenses will increase substantially if and as the Corporation:
| · | initiates or continues the clinical trials of DPX Survivac and other product candidates; |
| · | seeks regulatory approvals for the product candidates that successfully complete clinical trials; |
| · | establishes a sales, marketing and distribution infrastructure to commercialize products for which the Corporation may obtain regulatory approval; |
| · | maintains, expands and protects the Corporation’s intellectual property portfolio; |
| · | continues other research and development efforts; |
| · | hires additional clinical, quality control, scientific and management personnel; and |
| · | adds operational, financial and management information systems and personnel, including personnel to support product development and planned commercialization efforts. |
To become and remain profitable, the Corporation must develop and eventually commercialize a product or products with significant market potential. This development and commercialization will require the Corporation to be successful in a range of challenging activities, including successfully completing preclinical testing and clinical trials of the product candidates, obtaining regulatory approval for these product candidates and marketing and selling those products that obtain regulatory approval. The Corporation is only in the preliminary stages of some of these activities. The Corporation may never succeed in these activities and may never generate revenues that are significant or large enough to achieve profitability. Even if profitability is achieved, the Corporation may not be able to sustain or increase profitability on a quarterly or annual basis. Failure to become and remain profitable would decrease the value of the Corporation and could impair the Corporation’s ability to raise capital, expand the business, maintain research and development efforts or continue operations. A decline in the value of the Corporation could also cause shareholders to lose all or part of their investment.
Risks Relating to this Offering
Management will have broad discretion as to the use of the proceeds from the Offering, and may not use the proceeds effectively.
Management of the Corporation will have broad discretion in the application of the net proceeds from the Offering and could spend the proceeds in ways that do not improve the results of operations of the Corporation or enhance the value of the Common Shares. Failure to apply these funds effectively could have a material adverse effect on the business of the Corporation, delay the development of its product candidates, and cause the price of the Common Shares to decline.
The market price of the Common Shares has been and is likely to continue to be volatile and an investment in Common Shares may suffer a decline in value.
You should consider an investment in Common Shares as risky and invest only if you can withstand a significant loss and wide fluctuations in the market value of your investment. The Corporation receives only limited attention by securities analysts and frequently experiences an imbalance between supply and demand for Common Shares. The market price of the Common Shares has been highly volatile and is likely to continue to be volatile. This leads to a heightened risk of securities litigation pertaining to such volatility. Factors such as the financial position of the Corporation and the ability of the Corporation to continue as a going concern; the ability to raise additional capital; the progress of the clinical trials; the ability to obtain partners and collaborators to assist with the future development of the products; general market conditions; announcements of technological innovations or new product candidates by the Corporation, the Corporation collaborators or its competitors; published reports by securities analysts; developments in patent or other intellectual property rights; public concern as to the safety and efficacy of drugs that the Corporation and its competitors develop; and shareholder interest in the Common Shares all contribute to the volatility of the market price of the Common Shares.
| S-29 |
Future sales of Common Shares by the Corporation or by its existing shareholders could cause the market price of the Common Shares to fall.
The issuance of Common Shares by the Corporation could result in significant dilution in the equity interest of existing shareholders and adversely affect the market price of the Common Shares. Sales by existing shareholders of a large number of Common Shares in the public market and the issuance of shares issued in connection with strategic alliances, or the perception that such additional sales could occur, could cause the market price of the Common Shares to decline and have an undesirable impact on the Corporation’s ability to raise capital. With any additional sale or issuance of Common Shares, investors will suffer dilution to their voting power and the Corporation may experience dilution in its earnings per Common Share.
Dilution of purchasers.
Purchasers who purchase Offered Shares as part of the Offering may pay more for the Offered Shares than the amounts paid by existing shareholders or security holders of the Corporation for their Common Shares. As a result, such purchasers may incur immediate and substantial dilution. Convertible securities have been issued and may be issued in the future by the Corporation at a lower price than the current market value of the Common Shares, consequently, purchasers who purchase Offered Shares under the Offering may incur substantial dilution in the near future.
There is no assurance of a sufficient liquid trading market for the Common Shares in the future.
Shareholders of the Corporation may be unable to sell significant quantities of Common Shares into the public trading markets without a significant reduction in the price of their Common Shares, or at all. There can be no assurance that there will be sufficient liquidity of the Common Shares on the trading market, and that the Corporation will continue to meet the listing requirements of the TSX or NASDAQ or achieve listing on any other public listing exchange.
No dividends have been paid on the Common Shares and the Corporation does not intend to pay dividends in the foreseeable future although it may ultimately do so in the appropriate circumstances.
The Corporation has paid no cash dividends on any of its Common Shares to date and currently intends to retain its future earnings, if any, to fund the development growth of its businesses. In addition, the terms of any future debt or credit facility may preclude the Corporation from paying any dividends unless certain consents are obtained and certain conditions are met.
As a passive foreign investment company (“PFIC”) for United States federal income tax purposes, certain adverse tax rules may apply to U.S. Holders of the Common Shares.
Based on estimates of the composition of the Corporation’s income and the value of its assets, the Corporation believes that it is a PFIC for United States federal income tax purposes for the 2017 taxable year and that it is likely to be a PFIC for the 2018 taxable year.
The Corporation will be classified as a PFIC for any taxable year for United States federal income tax purposes if either (i) 75% or more of its gross income in that taxable year is passive income or (ii) the average percentage of its assets by value in that taxable year which produce or are held for the production of passive income (which includes cash) is at least 50%.
PFIC status is determined annually and depends upon the composition of a company’s income and assets and the market value of its stock from time to time. Therefore, there can be no assurance as to the Corporation’s PFIC status for future taxable years. The value of the Corporation’s assets will be based, in part, on the then market value of its Common Shares, which is subject to change.
If the Corporation is a PFIC for any taxable year during which a U.S. Holder (as defined under “Certain U.S. Federal Income Tax Considerations” in this prospectus) holds Common Shares, such U.S. Holders could be subject to adverse United States federal income tax consequences whether or not the Corporation continues to be a PFIC. For example, U.S. Holders may become subject to increased tax liabilities under United States federal income tax laws and regulations, and will become subject to burdensome reporting requirements. If the Corporation is a PFIC during a taxable year which a U.S. Holder holds Common Shares, such U.S. Holder may be able to make a “mark-to-market” election or a “qualified electing fund” election that could mitigate the adverse United States federal income tax consequences that would otherwise apply to such U.S. Holder. Although upon request of a U.S. Holder, the Corporation will provide the information necessary for a U.S. Holder to make the qualified electing fund election, no assurance can be given that such information will be available for any lower-tier PFIC that the Corporation does not control. See “Certain U.S. Federal Income Tax Considerations” for additional information.
U.S. Holders are urged to consult their own tax advisers as to the United State federal income tax consequences related to the Corporation’s classification as a PFIC.
| S-30 |
Certain Canadian Federal Income Tax Considerations
In the opinion of McCarthy Tétrault LLP, Canadian counsel to the Corporation, and Blake, Cassels & Graydon, LLP, Canadian counsel to the Underwriters, the following is, as of the date of this Prospectus Supplement, a summary of the principal Canadian federal income tax considerations under the Income Tax Act (Canada) (“Tax Act”) and the regulations thereunder generally applicable to an investor who acquires as beneficial owner Offered Shares pursuant to the Offering and who, for the purposes of the Tax Act and at all relevant times, deals at arm’s length with the Corporation and each of the Underwriters, is not affiliated with the Corporation or any of the Underwriters, is not exempt from tax under Part I of the Tax Act, and who acquires and holds the Offered Shares as capital property (a “Holder”). Generally, the Offered Shares will be considered to be capital property to a Holder thereof provided that the Holder does not use the Offered Shares in the course of carrying on a business of trading or dealing in securities and such Holder has not acquired them or been deemed to have acquired them in one or more transactions considered to be an adventure or concern in the nature of trade.
This summary does not apply to a Holder: (i) that is a “financial institution” for the purposes of the “mark-to-market property rules” of the Tax Act; (ii) that is a “specified financial institution” (as defined in the Tax Act); (iii) an interest in which would be a “tax shelter investment” as defined in the Tax Act; (iv) that has made a “functional currency” reporting election under the Tax Act to determine its “Canadian tax results” (as defined in the Tax Act) in a currency other than Canadian currency; (v) that has or will enter into a “synthetic disposition arrangement” or “derivative forward agreement” (as such terms are defined in the Tax Act); or (vi) that is a corporation resident in Canada and is, or becomes as part of a transaction or event or a series of transactions or events that includes the acquisition of an Offered Share, controlled by a non-resident corporation for purposes of section 212.3 of the Tax Act. Such Holders should consult their own tax advisors with respect to an investment in Offered Shares.
This summary is based upon the current provisions of the Tax Act and the Regulations in force as of the date hereof and counsel’s understanding of the administrative policies and assessing practices of the Canada Revenue Agency (the “CRA”) published in writing by the CRA prior to the date hereof. This summary takes into account all specific proposals to amend the Tax Act and the Regulations publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date hereof (the “Tax Proposals”) and assumes that the Tax Proposals will be enacted in the form proposed, although no assurance can be given that the Tax Proposals will be enacted in their current form or at all. Other than the Tax Proposals, this summary does not otherwise take into account any changes in law, whether by legislative, governmental, administrative or judicial decision or action, nor does it take into account or consider any provincial, territorial or foreign income tax considerations, which considerations may differ significantly from the Canadian federal income tax considerations discussed in this summary. This summary also does not take into account any change in the administrative policies or assessing practices of the CRA.
This summary is of a general nature only, is not exhaustive of all possible Canadian federal income tax considerations and is not intended to be, nor should it be construed to be, legal or tax advice to any particular Holder. Holders should consult their own tax advisors with respect to their particular circumstances.
Resident Holders
The following section of this summary applies to Holders who, for the purposes of the Tax Act are or are deemed to be resident in Canada at all relevant times (“Resident Holders”). Certain Resident Holders whose Offered Shares might not constitute capital property may make, in certain circumstances, an irrevocable election permitted by subsection 39(4) of the Tax Act to deem the Offered Shares, and every other “Canadian security” as defined in the Tax Act, held by such Resident Holders, in the taxation year of the election and each subsequent taxation year to be capital property. Resident Holders contemplating an election under subsection 39(4) of the Tax Act should consult their own tax advisors.
| S-31 |
Dividends
Dividends received or deemed to be received on the Offered Shares will be included in computing a Resident Holder’s income. In the case of a Resident Holder that is an individual (other than certain trusts), such dividends will be subject to the gross-up and dividend tax credit rules normally applicable in respect of “taxable dividends” received from “taxable Canadian corporations” (as each term is defined in the Tax Act). An enhanced gross-up and dividend tax credit will be available to individuals in respect of “eligible dividends” (as defined in the Tax Act) designated by the Corporation to the Resident Holder in accordance with the provisions of the Tax Act. There may be limitations under the Tax Act on the Corporation’s ability to designate a dividend as an “eligible dividend”, and the Corporation has made no commitments in this regard.
Dividends received or deemed to be received on an Offered Share held by a Resident Holder that is a corporation will be included in computing such Resident Holder’s income and will generally be deductible in computing its taxable income, subject to the provisions of the Tax Act. A Resident Holder that is a “private corporation” or a “subject corporation” (as each term is defined in the Tax Act) may be liable to pay an additional tax (refundable under certain circumstances) under Part IV of the Tax Act on dividends received or deemed to be received on an Offered Share to the extent that such dividends are deductible in computing the Resident Holder’s taxable income for the year.
In certain circumstances, subsection 55(2) of the Tax Act may treat a taxable dividend received or deemed to be received by a Resident Holder that is a corporation as proceeds of disposition or a capital gain. Resident Holders that are corporations should consult their own tax advisors with respect to the application of subsection 55(2) having regard to their own circumstances.
Disposition of Offered Shares
Upon a disposition (or a deemed disposition) of an Offered Share (other than to the Corporation unless purchased by the Corporation in the open market in the manner in which shares are normally purchased by any member of the public in the open market), a Resident Holder generally will realize a capital gain (or a capital loss) equal to the amount by which the proceeds of disposition of such security, as applicable, net of any reasonable costs of disposition, are greater (or are less) than the adjusted cost base of such security to the Resident Holder. The adjusted cost base to a Resident Holder of Offered Shares acquired pursuant to this Offering will be determined by averaging the cost of such Offered Shares with the adjusted cost base of all other Common Shares (if any) held by the Resident Holder as capital property at that time. The tax treatment of capital gains and capital losses is discussed in greater detail below under the subheading “Capital Gains and Capital Losses”.
Capital Gains and Capital Losses
Generally, a Resident Holder is required to include in computing its income for a taxation year one-half of the amount of any capital gain (a “taxable capital gain”) realized in the year. Subject to and in accordance with the provisions of the Tax Act, a Resident Holder is required to deduct one-half of the amount of any capital loss (an “allowable capital loss”) realized in a taxation year from taxable capital gains realized in the year by such Resident Holder. Allowable capital losses in excess of taxable capital gains may be carried back and deducted in any of the three preceding years or carried forward and deducted in any following taxation year against taxable capital gains realized in such year (but not against other income) to the extent and under the circumstances described in the Tax Act.
The amount of any capital loss realized on the disposition or deemed disposition of Offered Shares by a Resident Holder that is a corporation may be reduced by the amount of dividends received or deemed to have been received by it on such Offered Shares or shares substituted for such Offered Shares to the extent and under the circumstance specified by the Tax Act. Similar rules may apply where an Offered Share is owned by a partnership or trust of which a corporation, trust or partnership is a member or beneficiary, as the case may be. Resident Holders to whom these rules may be relevant should consult their own tax advisors.
| S-32 |
A Resident Holder that is throughout the relevant taxation year a “Canadian-controlled private corporation” (as defined in the Tax Act) may be liable to pay an additional tax (refundable under certain circumstances) on its “aggregate investment income” (as defined in the Tax Act) for the year, which includes taxable capital gains.
Minimum Tax
Capital gains realized and dividends received (or deemed to be received) by a Resident Holder that is an individual or a trust, other than certain specified trusts, may affect the Resident Holder’s liability to pay minimum tax under the Tax Act. Resident Holders should consult their own tax advisors with respect to the application of minimum tax.
Non-Resident Holders
The following section of this summary is generally applicable to Holders who for the purposes of the Tax Act and any applicable income tax treaty and at all relevant times, (i) are neither resident nor deemed to be resident in Canada and (ii) do not use or hold, and will not be deemed to use or hold, the Offered Shares in carrying on a business in Canada (“Non-Resident Holders”). This summary does not apply to a Non-Resident Holder that is (i) an insurer carrying on business in Canada and elsewhere or (ii) that is an “authorized foreign bank” (as defined in the Tax Act). Such Non-Resident Holders should consult their own tax advisors.
Currency
For purposes of the Tax Act, all amounts relating to the acquisition, holding or disposition of the Common Shares (including dividends, adjusted cost base and proceeds of disposition) must be expressed in Canadian dollars based on the exchange rate as quoted by the Bank of Canada for the applicable day or such other rate of exchange that is acceptable to the CRA.
Dividends
Dividends paid or credited or deemed to be paid or credited to a Non-Resident Holder by the Corporation are subject to Canadian withholding tax at the rate of 25% on the gross amount of the dividend unless such rate is reduced by the terms of an applicable tax treaty. For example, under the Canada-United States Tax Convention (1980), as amended (the “Treaty”), the rate of withholding tax on dividends paid or credited to a Non-Resident Holder beneficially owning the dividends who is resident in the U.S. for purposes of the Treaty and who is fully entitled to the benefits of the Treaty (a “U.S. Holder”) is generally limited to 15% of the gross amount of the dividend (or 5% in the case of a U.S. Holder that is a corporation beneficially owning at least 10% of the Corporation’s voting shares). Non-Resident Holders are urged to consult their own tax advisors to determine their entitlement to relief under an applicable income tax treaty.
Dispositions of Offered Shares
A Non-Resident Holder generally will not be subject to tax under the Tax Act in respect of a capital gain realized on the disposition or deemed disposition of an Offered Share, unless the Offered Share constitutes “taxable Canadian property” to the Non-Resident Holder thereof for purposes of the Tax Act, and the Non-Resident Holder is not entitled to relief under the terms of an applicable tax treaty. In addition, capital losses arising on the disposition or deemed disposition of an Offered Share will not be recognized under the Tax Act, unless the Offered Share constitutes “taxable Canadian property” to the Non-Resident Holder thereof for purposes of the Tax Act, and the Non-Resident Holder is not entitled to relief under the terms of an applicable tax treaty.
Provided the Common Shares are listed on a “designated stock exchange”, as defined in the Tax Act (which currently includes the TSX), at the time of disposition, the Offered Shares generally will not constitute taxable Canadian property of a Non-Resident Holder at that time, unless at any time during the 60 month period immediately preceding the disposition the following two conditions are met concurrently: (i) one or any combination of (a) the Non-Resident Holder, (b) persons with whom the Non-Resident Holder did not deal at arm’s length, and (c) partnerships in which the Non-Resident Holder or a person with whom the Non-Resident Holder did not deal at arm’s length held a membership interest directly or indirectly through one or more partnerships owned 25% or more of the issued shares of any class or series of shares of the Corporation; and (ii) more than 50% of the fair market value of the Common Shares of the Corporation was derived directly or indirectly from one or any combination of (a) real or immovable property situated in Canada, (b) “Canadian resource properties” (as defined in the Tax Act), (c) “timber resource properties” (as defined in the Tax Act) and (d) an option in respect of, an interest in, or for civil law rights in, any of the foregoing property, whether or not such property exists. Notwithstanding the foregoing, an Offered Share may in certain circumstances otherwise be deemed to be taxable Canadian property to a Non-Resident Holder for purposes of the Tax Act.
| S-33 |
A Non-Resident Holder’s capital gain (or capital loss) in respect of Offered Shares that constitute or are deemed to constitute taxable Canadian property (and are not “treaty-protected property” as defined in the Tax Act) will generally be computed in the manner described above under the subheading “Resident Holders – Disposition of Offered Shares” and such Non-Resident Holder may be required to report the disposition of such Offered Shares by filing a tax return in accordance with the Tax Act.
Non-Resident Holders whose Offered Shares are taxable Canadian property should consult their own tax advisors.
Certain U.S. Federal Income Tax Considerations
The following is a general summary of certain material U.S. federal income tax considerations relevant to a U.S. Holder (as defined below) arising from and relating to the acquisition, ownership, and disposition of Common Shares acquired pursuant to this Prospectus.
This summary is for general information purposes only and does not purport to be a complete analysis or listing of all potential U.S. federal income tax consequences that may apply to a U.S. Holder arising from and relating to the acquisition, ownership, and disposition of Common Shares. In addition, this summary does not take into account the individual facts and circumstances of any particular U.S. Holder that may affect the U.S. federal income tax consequences to such U.S. Holder. Accordingly, this summary is not intended to be, and should not be construed as, legal or U.S. federal income tax advice with respect to any U.S. Holder. Except as discussed below, this summary does not discuss applicable income tax reporting requirements. This summary does not address the U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences to U.S. Holders of the acquisition, ownership, and disposition of Common Shares. Each prospective U.S. Holder should consult its own tax advisor regarding the U.S. federal, U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences relating to the acquisition, ownership, and disposition of Common Shares.
No legal opinion from U.S. legal counsel or ruling from the Internal Revenue Service (the “IRS”) has been requested, or will be obtained, regarding the U.S. federal income tax consequences of the acquisition, ownership, and disposition of Common Shares. This summary is not binding on the IRS, and the IRS is not precluded from taking a position that is different from, and contrary to, the positions taken in this summary. In addition, because the authorities upon which this summary is based are subject to various interpretations, the IRS and the U.S. courts could disagree with one or more of the positions taken in this summary.
Scope of this Summary
Authorities
This summary is based on the Internal Revenue Code of 1986, as amended (the “Code”), Treasury Regulations (whether final, temporary, or proposed), published rulings of the IRS, published administrative positions of the IRS, the Convention Between Canada and the United States of America with Respect to Taxes on Income and on Capital, signed September 26, 1980, as amended (the “Canada-U.S. Tax Convention”), and U.S. court decisions that are applicable and, in each case, as in effect and available, as of the date of this document. Any of the authorities on which this summary is based could be changed in a material and adverse manner at any time, and any such change could be applied on a retroactive or prospective basis. This summary does not discuss the potential effects, whether adverse or beneficial, of any proposed legislation that, if enacted, could be applied on a retroactive or prospective basis.
| S-34 |
U.S. Holders
For purposes of this summary, the term “U.S. Holder” means a beneficial owner of Common Shares acquired pursuant to this Prospectus that is for U.S. federal income tax purposes:
| - | an individual who is a citizen or resident of the U.S.; |
| - | a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) organized under the laws of the U.S., any state thereof or the District of Columbia; |
| - | an estate whose income is subject to U.S. federal income taxation regardless of its source; or |
| - | a trust that (a) is subject to the primary supervision of a court within the U.S. and one or more U.S. persons has the authority to make all substantial decisions of the trust or (b) has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person. |
Non-U.S. Holders
For purposes of this summary, a “non-U.S. Holder” is a beneficial owner of Common Shares that is not a U.S. Holder and is not treated as a partnership for U.S. federal income tax purposes. This summary does not address the U.S. federal income tax consequences to non-U.S. Holders arising from and relating to the acquisition, ownership, and disposition of Common Shares. Accordingly, a non-U.S. Holder should consult its own tax advisor regarding the U.S. federal, U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and foreign tax consequences (including the potential application of and operation of any income tax treaties) relating to the acquisition, ownership, and disposition of Common Shares.
U.S. Holders Subject to Special U.S. Federal Income Tax Rules Not Addressed
This summary does not address the U.S. federal income tax considerations applicable to U.S. Holders that are subject to special provisions under the Code, including, but not limited to, the following: (a) U.S. Holders that are tax-exempt organizations, qualified retirement plans, individual retirement accounts, or other tax-deferred accounts; (b) U.S. Holders that are financial institutions, underwriters, insurance companies, real estate investment trusts, or regulated investment companies; (c) U.S. Holders that are broker-dealers, dealers, or traders in securities or currencies that elect to apply a mark-to-market accounting method; (d) U.S. Holders that have a “functional currency” other than the U.S. dollar; (e) U.S. Holders that own Common Shares as part of a straddle, hedging transaction, conversion transaction, constructive sale, or other arrangement involving more than one position; (f) U.S. Holders that acquired Common Shares in connection with the exercise of employee stock options or otherwise as compensation for services; (g) U.S. Holders that hold Common Shares other than as a capital asset within the meaning of Section 1221 of the Code (generally, property held for investment purposes); or (h) U.S. Holders that own, have owned or will own (directly, indirectly, or by attribution) 10% or more of the total combined voting power of the outstanding Common Shares of the Corporation. This summary also does not address the U.S. federal income tax considerations applicable to U.S. Holders who are: (a) U.S. expatriates or former long-term residents of the U.S.; (b) persons that have been, are, or will be a resident or deemed to be a resident in Canada for purposes of the Tax Act; (c) persons that use or hold, will use or hold, or that are or will be deemed to use or hold Common Shares in connection with carrying on a business in Canada; (d) persons whose Common Shares constitute “taxable Canadian property” under the Tax Act; or (e) persons that have a permanent establishment in Canada for the purposes of the Canada-U.S. Tax Convention. U.S. Holders that are subject to special provisions under the Code, including, but not limited to, U.S. Holders described immediately above, should consult their own tax advisor regarding the U.S. federal, U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences relating to the acquisition, ownership and disposition of Common Shares.
If an entity or arrangement that is classified as a partnership for U.S. federal income tax purposes holds Common Shares, the U.S. federal income tax consequences to such entity and the partners (or other owners) of such partnership generally will depend on the activities of the partnership and the status of such partners. This summary does not address the tax consequences to any such owner. Partners of entities or arrangements that are classified as partnerships for U.S. federal income tax purposes should consult their own tax advisors regarding the U.S. federal income tax consequences arising from and relating to the acquisition, ownership, and disposition of Common Shares.
| S-35 |
Ownership and Disposition of Common Shares
The following discussion is subject in its entirety to the rules described below under the heading “Passive Foreign Investment Company Rules”.
Taxation of Distributions
A U.S. Holder that receives a distribution, including a constructive distribution, with respect to a Common Share will be required to include the amount of such distribution in gross income as a dividend (without reduction for any Canadian or foreign income tax withheld from such distribution) to the extent of the current or accumulated “earnings and profits” of the Corporation, as computed for U.S. federal income tax purposes. To the extent that a distribution exceeds the current and accumulated “earnings and profits” of the Corporation, such distribution will be treated first as a tax-free return of capital to the extent of a U.S. Holder’s tax basis in the Common Shares and thereafter as gain from the sale or exchange of such Common Shares (see “Sale or Other Taxable Disposition of Common Shares”). However, the Corporation may not maintain the calculations of earnings and profits in accordance with U.S. federal income tax principles, and each U.S. Holder should therefore assume that any distribution by the Corporation with respect to the Common Shares will constitute ordinary dividend income. Dividends received on Common Shares generally will not be eligible for the “dividends received deduction” available to U.S. corporate shareholders receiving dividends from U.S. corporations.
Subject to applicable limitations and provided the Corporation is eligible for the benefits of the Canada – U.S. Tax Convention or the Common Shares are readily tradable on a United States securities market, dividends paid by the Corporation to non-corporate U.S. Holders, including individuals, generally will be eligible for the preferential tax rates applicable to long-term capital gains for dividends provided certain holding period and other conditions are satisfied, including that the Corporation not be classified as a PFIC in the tax year of distribution or in the preceding tax year. The dividend rules are complex, and each U.S. Holder should consult its own tax advisor regarding the application of such rules.
Sale or Other Taxable Disposition of Common Shares
A U.S. Holder will recognize gain or loss on the sale or other taxable disposition of Common Shares in an amount equal to the difference, if any, between (a) the amount of cash plus the fair market value of any property received and (b) such U.S. Holder’s tax basis in such Common Shares sold or otherwise disposed of. Any such gain or loss generally will be capital gain or loss, which will be long-term capital gain or loss if, at the time of the sale or other disposition, such Common Shares are held for more than one year.
Preferential tax rates apply to long-term capital gains of non-corporate U.S. Holders. There are currently no preferential tax rates for long-term capital gains of a U.S. Holder that is a corporation. Deductions for capital losses are subject to significant limitations under the Code. A U.S. Holder’s tax basis in Common Shares generally will be such U.S. Holder’s U.S. dollar cost for such Common Shares.
| S-36 |
Passive Foreign Investment Company
Based on the projected composition of the Corporation’s income, the Corporation believes that it is a PFIC for United States federal income tax purposes for the 2017 taxable year and that it is likely to be a PFIC for the 2018 taxable year. The determination of whether the Corporation will continue as a PFIC is made annually.
In general, the Corporation will be a PFIC for any taxable year in which:
| · | at least 75% of the Corporation’s gross income is passive income, or |
| · | at least 50% of the value (determined based on a quarterly average) of its assets is attributable to assets that produce or are held for the production of passive income. |
For this purpose, passive income generally includes dividends, interest, royalties and rents (other than royalties and rents derived in the active conduct of a trade or business and not derived from a related person). If the Corporation owns at least 25% (by value) of the stock of another corporation, it will be treated, for purposes of the PFIC tests, as owning its proportionate share of the other corporation’s assets and receiving its proportionate share of the other corporation’s income.
If the Corporation is a PFIC for any taxable year during which a U.S. Holder holds the Common Shares, the U.S. Holder will be subject to special tax rules with respect to any “excess distribution” received and any gain realized from a sale or other disposition, including a pledge, of the Common Shares. Distributions received in a taxable year that are greater than 125% of the average annual distributions received during the shorter of the three preceding taxable years or a U.S. Holder’s holding period for the Common Shares will be treated as excess distributions. Under these special tax rules:
| · | the excess distribution or gain will be allocated ratably over a U.S. Holder’s holding period for the Common Shares, |
| · | the amount allocated to the current taxable year, and any taxable year prior to the first taxable year in which the Corporation was a PFIC, will be treated as ordinary income, and |
| · | the amount allocated to each other year will be subject to tax at the highest ordinary income tax rate in effect for that year and the interest charge generally applicable to underpayments of tax will be imposed on the resulting tax attributable to each such year. |
If the Corporation is a PFIC for any year during which a U.S. Holder holds Common Shares, the Corporation will generally continue to be treated as a PFIC with respect to such holder for all subsequent years during which such Common Shares continue to be held, even if the Corporation ceases to meet the threshold requirements for PFIC status. U.S. Holders should consult with their own tax advisors regarding the availability of a “deemed sale” election that in certain circumstances would allow such holder to terminate PFIC status with respect to such Common Shares.
If the Corporation is a PFIC for any taxable year during which a U.S. Holder holds Common Shares and any of the Corporation’s non-U.S. subsidiaries is also a PFIC, or a lower-tier PFIC, a U.S. Holder would be treated as owning a proportionate amount (by value) of the shares of the lower-tier PFIC for purposes of the application of these rules on (i) excess distributions by the lower-tier PFIC, and (ii) a disposition of shares of a lower-tier PFIC, in each case as if the U.S. Holder held such shares directly, even though the holders have not received the proceeds of those distributions or dispositions directly.
| S-37 |
In lieu of being subject to the excess distribution rules discussed above with respect to the Common Shares (but not with respect to any lower-tier PFIC), a U.S. Holder may make an election to include gain on the stock of a PFIC as ordinary income under a mark-to-market method, provided that such stock is regularly traded on a qualified exchange. The Common Shares will be treated as “regularly traded” in any calendar year in which more than a de minimis quantity of such shares are traded on a qualified exchange on at least 15 days during each calendar quarter of such year. The NASDAQ on which the Common Shares are expected to be traded is a qualified exchange for this purpose.
If a U.S. Holder makes an effective mark-to-market election, it will include in each year the Corporation is a PFIC as ordinary income the excess of the fair market value of such U.S. Holder’s Common Shares at the end of the year over the U.S. Holder’s adjusted tax basis in the Common Shares. A U.S. Holder will be entitled to deduct as an ordinary loss in each such year the excess of the U.S. Holder’s adjusted tax basis in the Common Shares over their fair market value at the end of the year, but only to the extent of the net amount previously included in income as a result of the mark-to-market election. If a U.S. Holder makes an effective mark-to-market election, any gain the U.S. Holder recognizes upon the sale or other disposition of its Common Shares of in a year that the Corporation is a PFIC it will be treated as ordinary income and any loss will be treated as ordinary loss, but only to the extent of the net amount of previously included income as a result of the mark-to-market election.
A U.S. Holder’s adjusted tax basis in its Common Shares will be increased by the amount of any income inclusion and decreased by the amount of any deductions under the mark-to-market rules. If a U.S. Holder makes a mark-to-market election, it will be effective for the taxable year for which the election is made and all subsequent taxable years that the Corporation is a PFIC unless the Common Shares are no longer regularly traded on a qualified exchange or the IRS consents to the revocation of the election. U.S. Holders are urged to consult their tax advisors about the availability of the mark-to-market election, and whether making the election would be advisable in their particular circumstances.
Alternatively, a U.S. Holder may avoid the rules described above by electing to treat the Corporation (and any lower-tier PFIC) as a “qualified electing fund,” or QEF, under Section 1295 of the Code. A QEF election requires a U.S. Holder to include currently in income each year its pro rata share of a PFIC’s ordinary earnings and net capital gains, regardless of whether or not such ordinary earnings and gains are actually distributed. Thus, a U.S. Holder could have a tax liability with respect to such ordinary earnings or gains without a corresponding receipt of cash. A U.S. Holder’s basis in the shares of a QEF will be increased to reflect the amount of the taxed but undistributed income. Distributions of income that had previously been taxed will result in a corresponding reduction of basis in the shares and will not be taxed again as a distribution to the U.S. Holder. In addition, a U.S. Holder will recognize capital gain or loss on the disposition of Common Shares in an amount equal to the difference between the amount realized and the holder’s adjusted tax basis in the Common Shares. To make a QEF election, a U.S. Holder will need to have an annual information statement from the PFIC setting forth the earnings and capital gains for the year. U.S. Holders should consult their own tax advisors as to the consequences of making a protective QEF election or other consequences of the QEF election. Upon request of a U.S. Holder, the Corporation will provide the information necessary for a U.S. Holder to make the QEF election. However, no assurance can be given that such QEF information will be available for any lower-tier PFIC that the Corporation does not control.
U.S. Holders will be required to file IRS Form 8621 if they hold the Common Shares in any year in which the Corporation is classified as a PFIC.
U.S. Holders are urged to consult their tax advisors concerning the United States federal income tax consequences of holding the Common Shares if the Corporation or its subsidiaries are considered a PFIC in any taxable year and concerning the consequences of making a protective QEF election or other consequences of the QEF election or any other United States federal income tax election described herein.
| S-38 |
Additional Considerations
Additional Tax on Passive Income
Individuals, estates and certain trusts whose income exceeds certain thresholds will be required to pay a 3.8% Medicare surtax on “net investment income” including, among other things, dividends and net gain from disposition of property (other than property held in certain trades or businesses). Special rules apply to PFICs. U.S. Holders should consult with their own tax advisors regarding the effect, if any, of this tax on their ownership and disposition of Common Shares.
Receipt of Foreign Currency
The amount of any distribution paid in Canadian dollars to a U.S. Holder in connection with the ownership of the Common Shares, or on the sale, exchange or other taxable disposition of Common Shares, will be included in the gross income of a U.S. Holder as translated into U.S. dollars calculated by reference to the exchange rate prevailing on the date of actual or constructive receipt of the payment, regardless of whether the Canadian dollars are converted into U.S. dollars at that time. If the Canadian dollars received are not converted into U.S. dollars on the date of receipt, a U.S. Holder will have a basis in the Canadian dollars equal to their U.S. dollar value on the date of receipt. Any U.S. Holder who receives payment in Canadian dollars and engages in a subsequent conversion or other disposition of the Canadian dollars may have a foreign currency exchange gain or loss that would be treated as ordinary income or loss, and generally will be U.S. source income or loss for foreign tax credit purposes. Different rules apply to U.S. Holders who use the accrual method with respect to foreign currency. Each U.S. Holder should consult its own U.S. tax advisor regarding the U.S. federal income tax consequences of receiving, owning, and disposing of Canadian dollars.
Foreign Tax Credit
Subject to the PFIC rules discussed above, a U.S. Holder that pays (whether directly or through withholding) Canadian income tax with respect to dividends paid on the Common Shares generally will be entitled, at the election of such U.S. Holder, to receive either a deduction or a credit for such Canadian income tax paid. Generally, a credit will reduce a U.S. Holder’s U.S. federal income tax liability on a dollar-for-dollar basis, whereas a deduction will reduce a U.S. Holder’s income subject to U.S. federal income tax. This election is made on a year-by-year basis and applies to all foreign taxes paid (whether directly or through withholding) by a U.S. Holder during a year. Complex limitations apply to the foreign tax credit, including the general limitation that the credit cannot exceed the proportionate share of a U.S. Holder’s U.S. federal income tax liability that such U.S. Holder’s “foreign source” taxable income bears to such U.S. Holder’s worldwide taxable income. In applying this limitation, a U.S. Holder’s various items of income and deduction must be classified, under complex rules, as either “foreign source” or “U.S. source”. Generally, dividends paid by a foreign corporation should be treated as foreign source for this purpose, and gains recognized on the sale of stock of a foreign corporation by a U.S. Holder should be treated as U.S. source for this purpose, except as otherwise provided in an applicable income tax treaty, and if an election is properly made under the Code. However, the amount of a distribution with respect to the Common Shares that is treated as a “dividend” may be lower for U.S. federal income tax purposes than it is for Canadian federal income tax purposes, resulting in a reduced foreign tax credit allowance to a U.S. Holder. In addition, this limitation is calculated separately with respect to specific categories of income. The foreign tax credit rules are complex, and each U.S. Holder should consult its own U.S. tax advisor regarding the foreign tax credit rules.
Special rules apply to the amount of foreign tax credit that a U.S. Holder may claim on a distribution from a PFIC. Subject to such special rules, non-U.S. taxes paid with respect to any distribution in respect of stock in a PFIC are generally eligible for the foreign tax credit. The rules relating to distributions by a PFIC and their eligibility for the foreign tax credit are complex, and a U.S. Holder should consult its own tax advisor regarding their application to the U.S. Holder.
| S-39 |
Backup Withholding and Information Reporting
Under U.S. federal income tax law and Treasury Regulations, certain categories of U.S. Holders must file information returns with respect to their investment in, or involvement in, a non-U.S. corporation. For example, U.S. return disclosure obligations (and related penalties) are imposed on U.S. Holders that hold certain specified foreign financial assets in excess of certain threshold amounts. The definition of “specified foreign financial assets” includes not only financial accounts maintained in foreign financial institutions, but also, if held for investment and not in an account maintained by certain financial institutions, any stock or security issued by a non-U.S. person, any financial instrument or contract held for investment that has an issuer or counterparty other than a U.S. person and any interest in a foreign entity. U.S. Holders may be subject to these reporting requirements unless their Common Shares are held in an account at certain financial institutions. Penalties for failure to file certain of these information returns are substantial. U.S. Holders should consult with their own tax advisors regarding the requirements of filing information returns on IRS Form 8938.
Payments made within the U.S. or by a U.S. payor or U.S. middleman of (a) distributions on the Common Shares, and (b) proceeds arising from the sale or other taxable disposition of Common Shares generally will be subject to information reporting. In addition, backup withholding, currently at a rate of 24% for the 2018 to 2025 tax years (increasing to 28% for tax years after 2025), may apply to such payments if a U.S. Holder (a) fails to furnish such U.S. Holder’s correct U.S. taxpayer identification number (generally on IRS Form W-9), (b) furnishes an incorrect U.S. taxpayer identification number, (c) is notified by the IRS that such U.S. Holder has previously failed to properly report items subject to backup withholding, or (d) fails to certify, under penalty of perjury, that such U.S. Holder has furnished its correct U.S. taxpayer identification number and that the IRS has not notified such U.S. Holder that it is subject to backup withholding. Certain exempt persons generally are excluded from these information reporting and backup withholding rules. Backup withholding is not an additional tax. Any amounts withheld under the U.S. backup withholding rules will be allowed as a credit against a U.S. Holder’s U.S. federal income tax liability, if any, or will be refunded, if such U.S. Holder furnishes required information to the IRS in a timely manner. The information reporting and backup withholding rules may apply even if, under the Canada-U.S. Tax Convention, payments are exempt from the dividend withholding tax or otherwise eligible for a reduced withholding rate. This discussion of reporting requirements set forth above is not intended to constitute an exhaustive description of all reporting requirements that may apply to a U.S. Holder. A failure to satisfy certain reporting requirements may result in an extension of the time period during which the IRS can assess a tax, and under certain circumstances, such an extension may apply to assessments of amounts unrelated to any unsatisfied reporting requirements. Each U.S. Holder should consult its own tax advisor regarding the information reporting and backup withholding rules.
THE ABOVE SUMMARY IS NOT INTENDED TO CONSTITUTE A COMPLETE ANALYSIS OF ALL U.S. TAX CONSIDERATIONS APPLICABLE TO U.S. HOLDERS WITH RESPECT TO THE OWNERSHIP AND DISPOSITION OF COMMON SHARES. U.S. HOLDERS SHOULD CONSULT THEIR OWN TAX ADVISORS AS TO THE TAX CONSIDERATIONS APPLICABLE TO THEM IN THEIR PARTICULAR CIRCUMSTANCES.
In the opinion of McCarthy Tétrault LLP, Canadian counsel to the Corporation, and Blake, Cassels & Graydon LLP, Canadian counsel to the Underwriters, based on the provisions of the Tax Act, as of the date hereof, the Offered Shares, if issued on the date hereof, would be “qualified investments” under the Tax Act for trusts governed by registered retirement savings plans (“RRSPs”), registered retirement income funds (“RRIFs”), registered education savings plans (“RESPs”), registered disability savings plans (“RDSPs”), deferred profit sharing plans, and tax-free savings accounts (“TFSAs”), provided that the Common Shares are listed on a “designated stock exchange” as defined in the Tax Act (which currently includes the TSX).
| S-40 |
Notwithstanding the foregoing, if an Offered Share held by a TFSA, RRSP, RRIF, RESP or RDSP is a “prohibited investment” for purposes of the Tax Act, the holder of the TFSA or RDSP, the annuitant of the RRSP or RRIF, or the subscriber of the RESP, as the case may be, will be subject to a penalty tax as set out in the Tax Act. An Offered Share will be a “prohibited investment” if the holder of the TFSA or RDSP, the annuitant of the RRSP or RRIF, or the subscriber of the RESP, as the case may be: (i) does not deal at arm’s length with the Corporation for the purposes of the Tax Act; or (ii) has a “significant interest” (within the meaning of the Tax Act) in the Corporation. In addition, an Offered Share will generally not be a “prohibited investment” if the Offered Share is “excluded property” (as defined in the Tax Act), for trusts governed by a TFSA, RRSP, RRIF, RESP or RDSP. Prospective investors who may wish to hold their Offered Shares in a trust governed by a TFSA, RRSP, RRIF, RESP or RDSP are advised to consult their own tax advisors regarding the “prohibited investment” rules having regard to their own particular circumstances.
Holders who intend to hold Offered Shares in a TFSA, RRSP, RRIF, RDSP or RESP should consult their own tax advisors.
Certain Canadian legal matters relating to the Offering will be passed upon on behalf of the Corporation by McCarthy Tétrault LLP and on behalf of the Underwriters by Blake, Cassels & Graydon LLP. As of the date hereof, the partners and associates of McCarthy Tétrault LLP, as a group, and the partners and associates of Blake, Cassels & Graydon LLP, as a group, beneficially own, directly or indirectly, less than 1% of the outstanding Common Shares.
Certain legal matters relating to United States law will be passed upon on behalf of the Corporation by Troutman Sanders LLP and on behalf of the Underwriters by Cooley LLP.
AUDITOR, TRANSFER AGENT AND REGISTRAR
The auditor of the Corporation is PricewaterhouseCoopers LLP, Chartered Professional Accountants, Halifax, Nova Scotia, Canada. PricewaterhouseCoopers LLP has confirmed that they are independent with respect to the Corporation within the meaning of the relevant rules and related interpretations prescribed by the relevant professional bodies in Canada and any applicable legislation or regulation and within the meaning of the U.S. Securities and Exchange Commission.
The transfer agent and registrar for the Common Shares is Computershare Investor Services Inc., at its principal offices located in Toronto, Ontario, Canada or Montreal, Quebec, Canada.
Julia Gregory, Wayne Pisano, Albert Scardino and Markus Warmuth, directors of the Corporation, all reside outside of Canada and have appointed IMV Inc., 130 Eileen Stubbs Avenue, Suite 19, Dartmouth, Nova Scotia, Canada, B3B 2C4, as agent for service of process.
Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process.
STATUTORY RIGHTS OF WITHDRAWAL AND RESCISSION
Securities legislation in certain of the provinces of Canada provides purchasers with the right to withdraw from an agreement to purchase securities. This right may be exercised within two business days after receipt or deemed receipt of a prospectus and any amendment. In several of the provinces of Canada, the securities legislation further provides a purchaser with remedies for rescission or, in some jurisdictions, revisions of the price or damages if the prospectus and any amendment contains a misrepresentation or is not delivered to the purchaser, provided that the remedies for rescission, revision of the price or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province for the particulars of these rights or consult with a legal advisor. Rights and remedies may also be available to purchasers under U.S. law; purchasers may wish to consult with a U.S. lawyer for particulars of these rights.
| S-41 |
The Corporation is incorporated under, and governed by, the laws of Canada. Many of its officers and directors and experts named in this Prospectus Supplement and the Base Shelf Prospectus are resident outside of the United States, and a majority of their assets, and the assets of IMV, are located outside the United States. As a result, it may be difficult for U.S. investors to effect service of process within the United States upon those directors, officers or experts who are not residents of the United States, or to realize in the United States upon judgments of courts of the United States predicated upon civil liability of such directors, officers or experts under U.S. federal securities laws.
IMV has filed with the SEC, concurrently with the filing of its U.S. Registration Statement on Form F-10 of which this Prospectus Supplement and the Base Shelf Prospectus form a part, an appointment of agent for service of process on Form F-X. Under the Form F-X, IMV appointed C T Corporation System as its agent for service of process in the United States in connection with any investigation or administrative proceeding conducted by the SEC, and any civil suit or action brought against or involving IMV in a U.S. court arising out of or related to or concerning the Offering of Offered Shares under the U.S. Registration Statement. However, it may be difficult for United States investors to effect service of process within the United States upon those officers or directors who are not residents of the United States, or to realize in the United States upon judgments of courts of the United States predicated upon the Corporation’s civil liability and the civil liability of such officers or directors under United States federal securities laws or the securities or “blue sky” laws of any state within the United States.
| S-42 |
CERTIFICATE OF THE CORPORATION
Dated: February 28, 2019
The short form prospectus, together with the documents incorporated in the prospectus by reference as supplement by the foregoing, constitutes full, true and plain disclosure of all material facts relating to the securities offered by the prospectus and this supplement as required by the securities legislation of British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Québec, Nova Scotia and Newfoundland and Labrador.
| /s/ Frederic Ors | /s/ Pierre Labbé | |||
| Frederic Ors Chief Executive Officer |
Pierre Labbé Chief Financial Officer |
| On behalf of the Board of Directors |
| /s/ Andrew J. Sheldon | /s/ James Hall | |||
| Andrew J. Sheldon Director |
James Hall Director |
| C-1 |
CERTIFICATE OF THE Canadian UNDERWRITERs
Dated: February 28, 2019
To the best of our knowledge, information and belief, the short form prospectus, together with the documents incorporated in the prospectus by reference, as supplemented by the foregoing constitutes full, true and plain disclosure of all material facts relating to the securities offered by the prospectus and this supplement, as required by the securities legislation of British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Québec, Nova Scotia and Newfoundland and Labrador.
| Wells Fargo Securities Canada, Ltd. | RAYMOND JAMES Ltd. | |
| /s/ Stephen Shapiro | /s/ Marwan Kubursi | |
| Stephen Shapiro Managing Director |
Marwan Kubursi Managing Director |
| C-2 |
Base Shelf Prospectus
This short form base shelf prospectus has been filed under legislation in the provinces British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Québec, Nova Scotia and Newfoundland and Labrador that permits certain information about these securities to be determined after this prospectus has become final and that permits the omission from this prospectus of that information. The legislation requires the delivery to purchasers of a prospectus supplement containing the omitted information within a specified period of time after agreeing to purchase any of these securities.
Information contained herein is subject to completion or amendment. A registration statement relating to these securities has been filed with the United States Securities and Exchange Commission. These securities may not be offered or sold nor may offers to buy be accepted prior to the time the registration statement becomes effective. This short form prospectus shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of these securities in any state in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state.
No securities regulatory authority has expressed an opinion about these securities and it is an offence to claim otherwise. This short form base shelf prospectus constitutes a public offering of these securities only in those jurisdictions where they may be lawfully offered for sale and therein only by persons permitted to sell such securities.
Information has been incorporated by reference in this short form prospectus from documents filed with securities commissions or similar authorities in Canada. Copies of the documents incorporated herein by reference may be obtained on request without charge from the Corporate Secretary of IMV Inc. at #53-1344 Summer Street, Suite 412, Halifax, Nova Scotia, Canada, B3H 0A8 (telephone (902) 492-1819), and are also available electronically at www.sedar.com.
SHORT FORM BASE SHELF PROSPECTUS
| New Issue | June 5, 2018 |

$150,000,000
Preferred Shares
Common Shares
Subscription Receipts
Warrants
Units
Under this short form base shelf prospectus (the “Prospectus”), IMV Inc. (formerly Immunovaccine Inc.) (“IMV” or the “Corporation”) may, from time to time during the 25-month period that this Prospectus, including any amendments, remains valid, offer and issue preferred shares (the “Preferred Shares”) or common shares (the “Common Shares”) of its share capital, or subscription receipts (the “Subscription Receipts”), warrants or options to purchase Common Shares (collectively, the “Warrants”) or units comprised of one or more of the other securities described in this Prospectus in any combination (the “Units” and together with the Common Shares, Subscription Receipts and Warrants, the “Securities”) in one or more offerings of up to $150,000,000 (or the equivalent in foreign currencies). The Securities may be offered separately or together, in amounts, at prices and on terms based on market conditions at the time of the sale and set forth in an accompanying prospectus supplement (a “Prospectus Supplement”). The Corporation may sell the Preferred Shares, the Subscription Receipts and the Warrants in one or more series.
The specific variable terms of any offering of Securities will be set forth in a Prospectus Supplement and may include, where applicable:
| - | in the case of Preferred Shares, the number of Preferred Shares offered, the offering price and any other specific terms; |
| - | in the case of Common Shares, the number of Common Shares offered, the offering price and any other specific terms; |
| - | in the case of Subscription Receipts, the number of Subscription Receipts offered, the issue price, the terms and procedures for the exchange of the Subscription Receipts and any other specific terms; |
| - | in the case of Warrants, the number of Warrants offered, the offering price, the designation, number and terms of the Securities that may be purchased upon exercise of each Warrant and any other specific terms; and |
| - | in the case of Units, the designation, number and terms of any other Securities comprising, in any combination, the Units. |
A Prospectus Supplement may include specific variable terms pertaining to the Securities that are not within the alternatives and parameters set forth in this Prospectus.
All shelf information permitted under Securities legislation to be omitted from this Prospectus will be contained in one or more Prospectus Supplements that will be delivered to purchasers together with this Prospectus. Each Prospectus Supplement will be incorporated by reference into this Prospectus for the purposes of Securities legislation as of the date of the Prospectus Supplement and only for the purposes of the distribution of the Securities to which the Prospectus Supplement pertains. This Prospectus and any applicable Prospectus Supplement should be read carefully before investing in Securities. This Prospectus may not be used to offer any of the Securities unless accompanied by a Prospectus Supplement.
The Common Shares are listed on the Toronto Stock Exchange (the “TSX”) under the symbol “IMV” and on the Nasdaq Capital Market (“NASDAQ”) under the symbol “IMV”. On June 4, 2018, the last trading day of the Common Shares on the TSX and the NASDAQ before the date hereof, the closing price of the Common Shares was $8.26 and US$6.879, respectively. Unless otherwise specified in an applicable Prospectus Supplement, the Preferred Shares, the Subscription Receipts, the Warrants and the Units will not be listed on any securities or stock exchange or on any automated dealer quotation system.
The Corporation may offer and sell Securities to or through underwriters, dealers, placement agents or other intermediaries and the Corporation may also offer and sell its Securities directly to one or more purchasers, or through agents designated from time to time at amounts and prices and other terms determined by the Corporation. The Prospectus Supplement relating to a particular offering of Securities will identify each underwriter, dealer, placement agent, intermediary or agent engaged in connection with the offering and sale of Securities and will set forth the plan of distribution for such Securities, including the proceeds to the Corporation and any fees, discounts, concessions or other compensation payable to the underwriters, dealers or agents, and any other material terms of the plan of distribution. See “Plan of Distribution”.
The offering of Securities hereunder is made by a Canadian issuer that is permitted, under a multijurisdictional disclosure system (“MJDS”) adopted by the United States and Canada, to prepare this Prospectus in accordance with Canadian disclosure requirements. Prospective investors should be aware that such requirements are different from those of the United States. Annual financial statements for the year ended December 31, 2017 included or incorporated herein have been prepared in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board (“IFRS”) and are subject to Canadian auditing and auditor independence standards and thus may not be comparable to financial statements of United States companies.
The enforcement by investors of civil liabilities under the United States federal Securities laws may be affected adversely by the fact that the Corporation is incorporated or organized under the laws of a foreign country, that some or all of its officers and directors may be residents of a foreign country, that some or all of the underwriters or experts named in this Prospectus or any Prospectus Supplement may by residents of a foreign country and that all or a substantial portion of the assets of the Corporation and said persons may be located outside the United States.
| ii |
THESE SECURITIES HAVE NOT BEEN APPROVED OR DISAPPROVED BY THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION (“SEC”) NOR HAS THE SECURITIES COMMISSION OF ANY STATE OF THE UNITED STATES OR ANY CANADIAN SECURITIES REGULATOR APPROVED OR DISAPPROVED THESE SECURITIES OR PASSED UPON THE ACCURACY OR ADEQUACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
Investors should be aware that the acquisition, holding or disposition of the Securities described herein may have tax consequences both in the United States and in Canada. Such consequences for investors who are resident in, or citizens of, the United States and Canada may not be described fully herein. You should read the tax discussion contained in the applicable Prospectus Supplement with respect to a particular offering of Securities and consult your own tax advisor with respect to your own particular circumstances.
Albert Scardino and Wayne Pisano, members of the board of directors of the Corporation, both reside outside of Canada and have appointed IMV Inc., #53-1344 Summer Street, Suite 412, Halifax, Nova Scotia, Canada, BH3 0A8, as agent for service of process. Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process.
The Corporation’s head office and registered office is located at #53-1344 Summer Street, Suite 412, Halifax, Nova Scotia, Canada, BH3 0A8.
Investing in the Securities involves risks, including those that are described in the “Risk Factors” section of this Prospectus. The Corporation will apply to list the Common Shares distributed under this Prospectus including the Common Shares underlying the Preferred Shares, Units, Warrants and Subscription Receipts, if any. However, unless specified in the applicable Prospectus Supplement, there is no market through which the Preferred Shares, Units, Warrants and Subscriptions Receipts may be sold and purchasers may not be able to resell the Preferred Shares, Units, Warrants and Subscription Receipts purchased under this Prospectus and the Prospectus Supplements. This may affect the pricing of the Preferred Shares, Units, Warrants and Subscription Receipts in the secondary market, the transparency and availability of trading prices, the liquidity of the Preferred Shares, Units, Warrants and Subscription Receipts and the extent of issuer regulation. See “Risk Factors”.
No underwriter, dealer, placement agent, other intermediary or agent has been involved in the preparation of this Prospectus or performed any review of the contents of this Prospectus.
On May 2, 2018, the Corporation filed articles of amendment to give effect to a consolidation of its Common Shares on the basis of 1 post-consolidation Common Share for each 3.2 pre-consolidation Common Shares. The post-consolidation Common Shares began trading on TSX on May 10, 2018. Historical trading prices and volumes have been amended to reflect the 3.2 for 1 consolidation.
The offering of Securities hereunder is subject to approval of certain legal matters on behalf of the Corporation by McCarthy Tétrault LLP, with respect to Canadian legal matters, and by Troutman Sanders LLP, with respect to U.S. legal matters.
| iii |
TABLE OF CONTENTS
Purchasers of Securities should rely only on the information contained in or incorporated by reference into this Prospectus or any applicable Prospectus Supplement. The Corporation has not authorized anyone to provide purchasers with different or additional information. If anyone provides purchasers with different or additional information, purchasers should not rely on it. The Corporation is not making an offer to sell or seeking an offer to buy these Securities in any jurisdiction where the offer or sale is not permitted. Purchasers should assume that the information contained in this Prospectus or any applicable Prospectus Supplement is accurate only as of the date on the front of those documents and that information contained in any document incorporated by reference is accurate only as of the date of that document, regardless of the time of delivery of this Prospectus or any applicable Prospectus Supplement or of any sale of the Securities. The Corporation’s business, financial condition, results of operations and prospects may have changed since those dates.
“DepoVax” is a trademark of the Corporation. This Prospectus also includes references to trade names and trademarks of other companies, which trade names and trademarks are the properties of their respective owners.
The corporate website of the Corporation is www.imv-inc.com. The information on the Corporation’s website is not intended to be included or incorporated by reference into this Prospectus and prospective purchasers should not rely on such information when deciding whether or not to invest in the Securities.
Statistical information and other data relating to the pharmaceutical and biotechnology industry included in this Prospectus are derived from recognized industry reports published by industry analysts, industry associations and/or independent consulting and data compilation organizations. Market data and industry forecasts used throughout this Prospectus were obtained from various publicly available sources. Although the Corporation believes that these independent sources are generally reliable, the accuracy and completeness of the information from such sources are not guaranteed and have not been independently verified.
In this Prospectus, unless otherwise noted, all dollar amounts are expressed in Canadian dollars.
This Prospectus is part of a registration statement on Form F-10 (the “U.S. Registration Statement”) relating to the Securities that the Corporation has or will file with the SEC. Under the U.S. Registration Statement, the Corporation may, from time to time, sell Securities described in this Prospectus in one or more offerings up to an aggregate offering amount of $150,000,000. This Prospectus, which constitutes part of the U.S. Registration Statement, provides you with a general description of the Securities that the Corporation may offer. Each time the Corporation sells Securities under the U.S. Registration Statement, it will provide a Prospectus Supplement that will contain specific information about the terms of that offering of Securities. A Prospectus Supplement may also add, update or change information contained in this Prospectus. Before you invest, you should read both this Prospectus and any applicable Prospectus Supplement together with additional information described under the heading “Documents Incorporated by Reference”. This Prospectus does not contain all of the information set forth in the U.S. Registration Statement, certain parts of which are omitted in accordance with the rules and regulations of the SEC, or the schedules or exhibits that are part of the U.S. Registration Statement. Investors in the United States should refer to the U.S. Registration Statement and the exhibits thereto for further information with respect to IMV and the Securities.
EXCHANGE RATE INFORMATION
The consolidated financial statements incorporated by reference into this Prospectus and the other documents incorporated by reference into this Prospectus, and the financial data derived from those consolidated financial statements included in this Prospectus, are presented in Canadian dollars, unless otherwise specified, and have been prepared in accordance with IFRS. References in this Prospectus to “dollars”, “Cdn$” or “$” are to Canadian dollars. United States dollars are indicated by the symbol “US$”.
The following table lists, for each period presented, the high and low exchange rates, the average of the exchange rates during the period indicated, and the exchange rates at the end of the period indicated, for one Canadian dollar, expressed in United States dollars, based on the closing exchange rate published by the Bank of Canada for the applicable periods.
| Year ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| High for the period | 0.8245 | 0.7977 | 0.8511 | |||||||||
| Low for the period | 0.7276 | 0.6869 | 0.7161 | |||||||||
| End of period | 0.7971 | 0.7448 | 0.7821 | |||||||||
| Average for the period | 0.7701 | 0.7550 | 0.7225 | |||||||||
On June 4, 2018, the closing exchange rate for one Canadian dollar, expressed in United States dollars, as reported by the Bank of Canada, was Cdn$1.00 = US$0.7735.
Cautionary Statement regarding Forward-Looking Statements
Certain statements contained in this Prospectus, any Prospectus Supplement and the documents incorporated by reference herein and therein may constitute “forward-looking information” within the meaning of applicable securities laws in Canada and “forward-looking statements” within the meaning of the United States Private Securities Litigation Reform Act of 1995 (collectively, “forward-looking statements”) which involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements of the Corporation, or industry results, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. When used in this Prospectus, such statements reflect current expectations regarding future events and operating performance and speak only as of the date of this Prospectus. Forward-looking statements may use such words as “will”, “may”, “could”, “intends”, “potential”, “plans”, “believes”, “expects”, “projects”, “estimates”, “anticipates”, “continue”, “potential”, “predicts” or “should” and other similar terminology.
Forward-looking statements include, but are not limited to, statements relating to:
| - | the Corporation’s business strategy; |
| - | statements with respect to the sufficiency of the Corporation’s financial resources to support its activities; |
| - | potential sources of funding; |
| - | the Corporation’s ability to obtain necessary funding on favorable terms or at all; |
| - | the Corporation’s expected expenditures and accumulated deficit level; |
| - | the Corporation’s expected outcomes from its ongoing and future research and research collaborations; |
| - | the Corporation’s exploration of opportunities to maximize shareholder value as part of the ordinary course of its business through collaborations, strategic partnerships and other transactions with third parties; |
| - | the Corporation’s plans for the research and development of certain product candidates; |
| - | the Corporation’s strategy for protecting its intellectual property; |
| - | the Corporation’s ability to identify licensable products or research suitable for licensing and commercialization; |
| - | the Corporation’s ability to obtain licences on commercially reasonable terms; |
| - | the Corporation’s plans for generating revenue; |
| - | the Corporation’s plans for future clinical trials; and |
| - | the Corporation’s hiring and retention of skilled staff. |
The forward-looking statements reflect the Corporation’s current views with respect to future events, are subject to risks and uncertainties, and are based upon a number of estimates and assumptions that, while considered reasonable by the Corporation, are inherently subject to significant business, economic, competitive, political and social uncertainties and contingencies. Many factors could cause the Corporation’s actual results, performance or achievements to be materially different from any future results, performance, or achievements that may be expressed or implied by such forward-looking statements, including, among others:
| 2 |
| - | obtaining additional funding on reasonable terms when necessary; |
| - | positive results of pre-clinical and clinical tests; |
| - | the Corporation’s ability to successfully develop existing and new products; |
| - | the Corporation’s ability to hire and retain skilled staff; |
| - | the products and technology offered by the Corporation’s competitors; |
| - | general business and economic conditions; |
| - | the Corporation’s ability to protect its intellectual property; |
| - | the Corporation’s ability to manufacture its products and to meet demand; and |
| - | regulatory approvals. |
Should one or more of these risks or uncertainties materialize, or should the assumptions set out in the section entitled “Risk Factors” underlying those forward-looking statements prove incorrect, actual results may vary materially from those described herein. These forward-looking statements are made as of the date of this Prospectus or, in the case of documents incorporated by reference in this Prospectus, as of the date of such documents, and the Corporation does not intend, and does not assume any obligation, to update these forward-looking statements, except as required by law. There is no assurance that such statements will prove to be accurate as actual results and future events could differ materially from those anticipated in such statements. Purchasers are cautioned that forward-looking statements are not guarantees of future performance and accordingly purchasers are cautioned not to put undue reliance on forward-looking statements due to the inherent uncertainty therein. New factors emerge from time to time, and it is not possible for management of the Corporation to predict all of these factors or to assess in advance the impact of each such factor on the Corporation’s business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statement.
The forward-looking statements contained in this Prospectus are expressly qualified by the foregoing cautionary statements and are made as of the date of this Prospectus. The Corporation does not undertake any obligation to publicly update or revise any forward-looking statements, except as required by applicable securities laws. Purchasers should read this Prospectus and consult their own professional advisors to assess the income tax, legal, risk factors and other aspects of their investment in the securities.
DOCUMENTS INCORPORATED BY REFERENCE
Information has been incorporated by reference in this Prospectus from documents filed with securities commissions or similar authorities in Canada. Copies of the documents incorporated herein by reference may be obtained on request without charge from the Corporate Secretary of the Corporation at #53-1344 Summer Street, Suite 412, Halifax, Nova Scotia, Canada, B3H 0A8 (telephone (902) 492-1819), and are also available electronically at www.sedar.com.
In addition to the continuous disclosure obligations of the Corporation under the securities laws of certain provinces of Canada, the Corporation is subject to certain of the information requirements of the U.S. Securities Exchange Act of 1934, as amended (the “Exchange Act”), and in accordance therewith file reports and other information with the SEC. Under MJDS, some reports and other information may be prepared in accordance with the disclosure requirements of Canada, which requirements are different from those of the United States. As a foreign private issuer, the Corporation is exempt from the rules under the Exchange Act prescribing the furnishing and content of proxy statements, and the Corporation’s officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, the Corporation may not be required to publish financial statements as promptly as U.S. companies. You may read any document that the Corporation files with or furnish to the SEC at the SEC’s public reference room at Room 1580, 100 F Street N.E., Washington, D.C. 20549. You may also obtain copies of the same documents from the public reference room of the SEC at 100 F Street N.E., Washington D.C. 20549 by paying a fee. You should call the SEC at 1-800-SEC-0330 or access its website at www.sec.gov for further information about the public reference room. As well, a free copy of any public document filed by IMV with the SEC’s Electronic Data Gathering and Retrieval (EDGAR) system is available from the SEC’s website at www.sec.gov.
| 3 |
The following documents filed with the securities commissions or similar authorities in the provinces of British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Québec, Nova Scotia and Newfoundland and Labrador are specifically incorporated by reference in and form an integral part of this Prospectus:
| (i) | the annual information form of the Corporation dated March 20, 2018 for the year ended December 31, 2017 (the “AIF”); |
| (ii) | the audited annual consolidated financial statements of the Corporation and the notes thereto for the years ended December 31, 2017 and 2016, together with the auditor’s report thereon; |
| (iii) | the management’s report on financial position and operating results of the Corporation for the year ended December 31, 2017 (the “Annual MD&A”), except for the “Letter to Shareholders” which is specifically excluded and is not incorporated by reference herein; |
| (iv) | the unaudited interim condensed consolidated financial statements of the Corporation and the notes thereto for the three months ended March 31, 2018 and 2017; |
| (v) | the management’s report on financial position and operating results of the Corporation for the three months ended March 31, 2018, except for the “Letter to Shareholders” which is specifically excluded and is not incorporated by reference herein; |
| (vi) | the management information circular dated March 29, 2018 relating to the annual and special meeting of shareholders of the Corporation held on May 1, 2018; |
| (vii) | the material change report dated February 2, 2018 relating to a bought-deal financing agreement to sell Common Shares (the “February 2018 Public Offering”); |
| (viii) | the material change report dated February 21, 2018 relating to the closing of the February 2018 Public Offering; and |
| (ix) | the material change report dated May 10, 2018 relating the NASDAQ listing, the consolidation of the Common Shares and the name change. |
Any documents of the Corporation of the type referred to in the preceding paragraph and any material change reports (excluding any confidential material change reports) filed by the Corporation with a securities commission or similar regulatory authority in Canada on or after the date of Prospectus and prior to the termination of the offering of Securities hereunder shall be deemed to be incorporated by reference into this Prospectus.
In addition, to the extent that any document or information incorporated by reference into this Prospectus is included in any report on Form 6-K, Form 40-F or Form 20-F (or any respective successor form) that is filed with or furnished to the SEC by the Corporation after the date of this Prospectus, such document or information shall be deemed to be incorporated by reference as an exhibit to the U.S. Registration Statement of which this Prospectus forms a part. In addition, the Corporation may incorporate by reference into this Prospectus, or the U.S. Registration Statement of which it forms a part, other information from documents that the Corporation will file with or furnish to the SEC pursuant to Section 13(a) or 15(d) of the Exchange Act, if and to the extent expressly provided therein.
Any statement contained in this Prospectus or in a document incorporated or deemed to be incorporated by reference in this Prospectus shall be deemed to be modified or superseded for purposes of this Prospectus to the extent that a statement contained herein or in any other subsequently filed document which also is or is deemed to be incorporated by reference in this Prospectus modifies or supersedes such statement. The modifying or superseding statement need not state that it has modified or superseded a prior statement or include any other information set forth in the document that it modifies or supersedes. Any statement so modified or superseded shall not be deemed to constitute a part of this Prospectus, except as so modified or superseded.
You should rely only on the information contained in or incorporated by reference in this Prospectus or any applicable Prospectus Supplement and on the other information included in the U.S. Registration Statement of which this Prospectus forms a part. The Corporation is not making an offer of Securities in any jurisdiction where the offer is not permitted by law.
| 4 |
DOCUMENTS FILED AS PART OF THE U.S. REGISTRATION STATEMENT
The following documents have been filed with the SEC as part of the U.S. Registration Statement of which this Prospectus is a part insofar as required by the SEC’s Form F-10:
| · | the documents listed under “Documents Incorporated by Reference” in this Prospectus; |
| · | the consent of PricewaterhouseCoopers LLP, the Corporation’s independent auditor; |
| · | the consent of McCarthy Tétrault LLP, the Corporation’s Canadian counsel; and |
| · | powers of attorney of the Corporation’s directors and officers, as applicable. |
A copy of the form of warrant indenture will be filed by post-effective amendment or by incorporation by reference to documents filed with or furnished to the SEC under the Exchange Act.
The Corporation was incorporated on May 18, 2007 under the name of Rhino Resources Inc. pursuant to the Canada Business Corporations Act. On September 28, 2009, the Corporation changed its name to Immunovaccine Inc. and consolidated its outstanding share capital on a 5 to 1 basis. On May 2, 2018, the Corporation changed its name to IMV Inc. and consolidated its outstanding share capital on a 3.2 to 1 basis.
The Corporation has one wholly-owned subsidiary, Immunovaccine Technologies Inc. (“IVT”), which is incorporated under the laws of Nova Scotia.
The Corporation’s head and registered office is located at 1344 Summer Street, Suite 412, Halifax, Nova Scotia, Canada, B3H 0A8.
Overview
IMV is a clinical-stage company pioneering a new class of immunotherapies based on a disruptive drug delivery technology (DPX) with potential applications in multiple markets in cancer, infectious diseases and other therapeutic areas. The DPX platform is based on a novel mechanism of action (MOA) for targeted delivery of active ingredients to immune cells using a patented lipid nanoparticle technology. The Corporation leverages this MOA to generate a new generation of therapeutic capabilities with a primary focus on T cell therapies for cancer.
The Corporation’s first cancer immunotherapy uses survivin-based peptides licensed from Merck KGaA, on a world-wide exclusive basis, formulated in DPX. Survivin is a well characterized and recognized tumor associated antigen known to be expressed during fetal development and across most tumour cell types, but is rarely present in normal, non-malignant adult cells. It has been shown that survivin was expressed in all 60 different human tumour lines used in the National Cancer Institute's cancer drug-screening program.
DPX-Survivac, is currently being tested in a co-funded Phase 1b clinical trial with Incyte Corporation (“Incyte”), which evaluates the combination of DPX-Survivac with Incyte’s investigational oral indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor, epacadostat, in ovarian cancer patients. DPX-Survivac is also being tested in two investigator-sponsored Phase 2 clinical trials in combination with checkpoint inhibitor pembrolizumab of Merck & Co Inc. in patients with recurrent, platinum-resistant and sensitive ovarian cancer and in patients with measurable or recurrent diffuse large B cell lymphoma (DLBCL). In infectious disease vaccine applications, the Corporation has completed a demonstration Phase 1 clinical trial with a target against the respiratory syncytial virus (RSV). The Corporation also has a commercial licencing agreement with Zoetis for the development of two cattle vaccines and is also conducting several research and clinical collaborations, including a collaboration with the Dana-Farber Cancer Institute for Human Papillomavirus (HPV) related cancers and with Leidos, Inc. in the United States for the development of vaccine candidates for malaria and the Zika virus.
| 5 |
Recent developments
On May 2, 2018, the Corporation filed articles of amendment to give effect to a consolidation of its Common Shares on the basis of 1 post-consolidation Common Share for each 3.2 pre-consolidation Common Shares. The post-consolidation Common Shares began trading on TSX on May 10, 2018.
The following table sets out certain information contained in documents incorporated by reference in this Prospectus for the periods indicated, restated as if the consolidation had occurred at such time. Due to the 3.2 for 1 share consolidation which occurred in May 2018, the total number of Common Shares and options outstanding, the total number of options exercisable and the weighted average Common Shares outstanding (basic and diluted) have all been divided by 3.2 from the numbers shown in the Corporation’s previously filed annual financial statements (reported on an annual basis). As a result of the consolidation, the basic and diluted earnings per Common Share have been multiplied by 3.2 from the numbers disclosed in the Corporation’s previously filed annual financial statements.
| December 31, 2017 | December 31, 2016 | |||||||
| Basic and diluted loss per share | (0.31 | ) | (0.28 | ) | ||||
| Common shares outstanding | 40,319,928 | 36,817,314 | ||||||
| Stock options outstanding | 1,498,044 | 1,961,765 | ||||||
| Warrants outstanding | 2,087,598 | 2,725,596 | ||||||
| Deferred share units outstanding | 186,327 | 101,563 | ||||||
| Weighted-average shares outstanding | 38,656,778 | 31,602,737 | ||||||
| Weighted-average shares outstanding - diluted | 38,656,778 | 31,602,737 | ||||||
On May 31, 2018, the Corporation announced that its Common Shares had been approved for listing on Nasdaq. Trading of the Common Shares on Nasdaq commenced on June 1, 2018 and concurrently the Common Shares ceased to be traded on the OTCQX.
On June 3, 2018, the Corporation announced that investigators shared new positive data in an oral presentation for its DeCidE1 (DPX-Survivac with low dose CyclophosphamIDe and Epacadostat) clinical study at the 2018 American Society for Clinical Oncology (ASCO) annual meeting. These data from the ongoing Phase 1b/2 trial evaluated the safety and efficacy of the combination of IMV’s lead candidate DPX-Survivac and low dose cyclophosphamide, with Incyte Corporation’s IDO1 enzyme inhibitor epacadostat, in patients with advanced recurrent ovarian cancer.
Except as otherwise disclosed in this Prospectus and the documents incorporated by reference herein, there have been no material changes in the consolidated share and loan capital of IMV from March 31, 2018 to the date of this Prospectus.
| 6 |
The aggregate proceeds of distributions of Securities under this Prospectus shall not exceed $150,000,000. The net proceeds to be received by the Corporation from the distribution from time to time of Securities under this Prospectus will be the gross proceeds of such issue less any commissions and expenses paid in connection therewith.
Unless otherwise specified in a Prospectus Supplement, the net proceeds received by the Corporation from the sale of the Securities will be used for working capital and general corporate purposes including, but not limited to, to advance the research and development and clinical advancement of the Corporation’s cancer and infectious disease vaccine candidates. A Prospectus Supplement will contain specific information about the use of proceeds from the sale of the Securities under that Prospectus Supplement.
More detailed information regarding the use of proceeds from the sale of the Securities will be described in any applicable Prospectus Supplement. Pending the application of the net proceeds, the Corporation intends to invest the net proceeds in investment-grade, interest-bearing securities, the primary objectives of which are liquidity and capital preservation.
Negative Cash Flow
The Corporation has incurred significant operating losses and negative cash flows from operations since inception and has an accumulated deficit of $70,820,867 as at December 31, 2017. The ability of the Corporation to continue as a going concern is dependent upon raising additional financing through equity and non-dilutive funding and partnerships. There can be no assurance that the Corporation will have sufficient capital to fund its ongoing operations, develop or commercialize any products without future financings. These material uncertainties cast significant doubt as to the Corporation’s ability to meet its obligations as they come due and, accordingly, the appropriateness of the use of accounting principles applicable to a going concern. If the Corporation is unable to obtain additional financing when required, the Corporation may have to substantially reduce or eliminate planned expenditures or the Corporation may be unable to continue operations.
The Corporation’s ability to continue as a going concern is dependent upon its ability to fund its research and development programs and defend its patent rights. It is expected that proceeds from the sale of Securities under the Prospectus will be used to fund anticipated negative cash flow from operating activities, as described above.
The Corporation may offer and sell its Securities to or through underwriters, dealers, placement agents or other intermediaries and the Corporation may also offer and sell its Securities directly to one or more purchasers or through agents in negotiated transactions, block trades, equity lines of credit or a combination of these methods, subject to obtaining any applicable exemption from registration requirements. The Securities offered pursuant to any Prospectus Supplement may be sold from time to time in one or more transactions at:
| - | a fixed price or prices, which may be changed from time to time; |
| - | market prices prevailing at the time of sale; |
| - | prices related to such prevailing market prices; or |
| - | other negotiated prices, including sales in transactions that are deemed to be “at-the-market” distribution” as defined in National Instrument 44-102 – Shelf Distributions, including sales made directly on the TSX, the NASDAQ or other existing trading markets for the Securities. |
The Corporation may only offer and sell the Securities pursuant to a Prospectus Supplement during the 25-month period that this Prospectus, including any amendments hereto, remains effective. The Prospectus Supplements for any of the Securities being offered thereby will set forth the terms of the offering of such Securities, including the type of Securities being offered, the name or names of any underwriters, dealers, placement agents, other intermediaries or agents, the purchase price of such Securities, the proceeds to the Corporation from such sale, any underwriting commissions or discounts and other items constituting compensation and any discounts or concessions allowed or re-allowed or paid to underwriters, dealers, placement agents, other intermediaries or agents. Only underwriters, dealers, placement agents, other intermediaries or agents so named in the Prospectus Supplements are deemed to be underwriters in connection with the Securities offered thereby.
| 7 |
In connection with the sale of Securities, underwriters, dealers, placement agents, other intermediaries or agents may receive compensation from the Corporation or from purchasers of Securities for whom they may act as intermediary or agents in the form of discounts, concessions or commissions. Underwriters, dealers, placement agents, other intermediaries or agents that participate in the distribution of Securities may be deemed to be underwriters and any discounts or commissions received by them from the Corporation and any profit on the resale of securities by them may be deemed to be underwriting discounts and commissions under applicable securities legislation.
If so indicated in the applicable Prospectus Supplements, the Corporation may authorize dealers, placement agents, other intermediaries or other persons acting as its agents to solicit offers by certain institutions to purchase the Securities directly from the Corporation pursuant to contracts providing for payment and delivery on a future date. These contracts will be subject only to the conditions set forth in the applicable Prospectus Supplements, which will also set forth the commission payable for solicitation of these contracts.
Any offering of Preferred Shares, Warrants, Units or Subscription Receipts will be a new issue of securities with no established trading market. Unless otherwise specified in the applicable Prospectus Supplements, the Preferred Shares, Warrants, Units or Subscription Receipts will not be listed on any securities or stock exchange or on any automated dealer quotation system. Unless otherwise specified in the applicable Prospectus Supplements, there is no market through which the Preferred Shares, Warrants, Units or Subscription Receipts may be sold and purchasers may not be able to resell Preferred Shares, Warrants, Units or Subscription Receipts purchased under this Prospectus or any Prospectus Supplement. This may affect the pricing of the Preferred Shares, Warrants, Units or Subscription Receipts in the secondary market, the transparency and availability of trading prices, the liquidity of the Securities, and the extent of issuer regulation. Certain dealers may make a market in the Preferred Shares, Warrants, Units or Subscription Receipts.
The Prospectus Supplements will set forth the terms of the offering of Securities, including:
| - | the name or the names of any underwriters, dealers, placement agents, other intermediaries or agents, if any; |
| - | the purchase price of, and form of consideration for, the Securities and the proceeds; |
| - | any delayed delivery arrangements; |
| - | any underwriting commissions, fees, discounts and other items constituting underwriters’ compensation; |
| - | the offering price; |
| - | any discounts or concessions allowed or re-allowed or paid to dealers; and |
| - | any other securities exchanges on which the Securities may be listed, if any. |
Only the underwriters, dealers, placement agents, other intermediaries or agents named in a Prospectus Supplement are deemed to be underwriters in connection with the Securities offered by that Prospectus Supplement.
The Common Shares may be sold, from time to time in one or more transactions at a fixed price or prices that may be changed or at market prices prevailing at the time of sale, at prices related to such prevailing market price or at negotiated prices.
Under agreements that may be entered into by IMV, underwriters, dealers, placement agents, other intermediaries or agents who participate in the distribution of Securities may be entitled to indemnification by the Corporation against certain liabilities, including liabilities under any applicable Canadian provincial securities legislation, or to contributions with respect to payments that such underwriters, dealers or agents may be required to make in that respect.
| 8 |
In connection with an offering, the underwriters, dealers, placement agents, other intermediaries or agents, if any, may over-allot or effect transactions that stabilize or maintain the market price of the Common Shares at a level above that which might otherwise prevail in the open market. Such transactions, if commenced, may be discontinued at any time and would be subject to applicable law.
By Underwriters, Dealers, Placement Agents or Other Intermediaries
If underwriters, dealers, placement agents or other intermediaries are used in the sale, the Securities will be acquired by such underwriters, dealers, placement agents or other intermediaries for their own account, as principals, and may be resold from time to time in one or more transactions, including negotiated transactions, at a fixed public offering price or at varying prices determined at the time of sale. Any public offering price and any discounts or concessions allowed or re-allowed or paid to underwriters, dealers, placement agents or other intermediaries may be changed from time to time. Unless otherwise set forth in the Prospectus Supplements relating thereto, the obligations of underwriters, dealers, placement agents or other intermediaries to purchase the Securities will be subject to certain conditions, but the underwriters, dealers, placement agents or other intermediaries will be obligated to purchase all of the Securities offered by the Prospectus Supplements if any of such Securities are purchased. The Corporation may agree to pay the underwriters, dealers, placement agents or other intermediaries a fee or commission for various services relating to the offering of any Securities. Any such fees or commissions will be paid out of the general corporate funds of the Corporation.
In compliance with the guidelines of the Financial Regulatory Authority Inc. (“FINRA”) and subject to the approval of FINRA, the maximum aggregate value of all compensation to be received by any FINRA member or independent broker-dealer will not exceed 8% of the gross proceeds from the sale of Securities pursuant to this Prospectus and any applicable Prospectus Supplement. If 5% or more of the net proceeds of any offering of Securities made under this Prospectus will be received by a FINRA member participating in the offering or affiliates or associated persons of such FINRA member, the offering will be conducted in accordance with FINRA Rule 5121 (or any successor rule).
By Agents
The Securities may also be sold through agents designated by the Corporation. Any agent involved will be named, and any fees or commissions payable by the Corporation to such agent will be set forth, in the applicable Prospectus Supplements. Any such fees or commissions will be paid out of the general corporate funds of the Corporation. Unless otherwise indicated in the Prospectus Supplements, any agent will be acting on a best efforts basis for the period of its appointment.
Direct Sales
Securities may also be sold directly by the Corporation at such prices and upon such terms as agreed to by the Corporation and the purchaser. In this case, no underwriters, dealers, placement agents, other intermediaries or agents would be involved in the offering.
General Information
Underwriters, dealers and agents that participate in the distribution of Securities may be deemed to be underwriters and any commissions received by them from the Corporation and any profit on the resale of Securities by them may be deemed to be underwriting commissions under the U.S. Securities Act of 1933, as amended.
Underwriters or agents who participate in the distribution of Securities may be entitled under agreements to be entered into with the Corporation to indemnification by the Corporation against certain liabilities, including liabilities under Canadian provincial and United States securities legislation, or to contribution with respect to payments which such underwriters or agents may be required to make in respect thereof. Such underwriters or agents may be customers of, engage in transactions with, or perform services for, us in the ordinary course of business.
| 9 |
The Corporation may enter into derivative transactions with third parties, or sell securities not covered by this Prospectus to third parties in privately negotiated transactions. If the applicable Prospectus Supplement indicates, in connection with those derivatives, the third parties may sell Securities covered by this Prospectus and the applicable Prospectus Supplement, including in short sale transactions. If so, the third parties may use Securities pledged by the Corporation or borrowed from the Corporation or others to settle those sales or to close out any related open borrowings of stock, and may use Securities received from the Corporation in settlement of those derivatives to close out any related open borrowings of stock. The third parties in such sale transactions will be identified in the applicable Prospectus Supplement.
One or more firms, referred to as “remarketing firms”, may also offer or sell the Securities, if the Prospectus Supplement so indicates, in connection with a remarketing arrangement upon their purchase. Remarketing firms will act as principals for their own accounts or as agents for the Corporation. These remarketing firms will offer or sell the Securities in accordance with the terms of the Securities. The Prospectus Supplement will identify any remarketing firm and the terms of its agreement, if any, with the Corporation and will describe the remarketing firm’s compensation. Remarketing firms may be deemed to be underwriters in connection with the Securities they remarket.
In connection with any offering of Securities, other than an “at-the-market” distribution, underwriters may over-allot or effect transactions which stabilize or maintain the market price of the Securities offered at a level above that which might otherwise prevail in the open market. Such transactions may be commenced, interrupted or discontinued at any time. With respect to an “at-the-market” distribution, no underwriter or dealer involved in the distribution, no affiliate of such an underwriter or dealer and no person or company acting jointly or in concert with such an underwriter or dealer will over-allot Securities in connection with the distribution or effect any other transactions that are intended to stabilize or maintain the market price of the Securities.
IMV’s authorized share capital consists of an unlimited number of Common Shares and Preferred Shares issuable in series, all without par value. As of June 4, 2018, a total of 43,221,570 Common Shares and no Preferred Shares are issued and outstanding.
On May 2, 2018, the Corporation filed articles of amendment to give effect to a consolidation of its Common Shares on the basis of 1 post-consolidation Common Share for each 3.2 pre-consolidation Common Shares.
Common Shares
The Common Shares of the Corporation rank junior to the Preferred Shares with respect to the payment of dividends, return of capital and distribution of assets in the event of liquidation, dissolution or winding-up of the Corporation. Subject to the prior rights of the holders of Preferred Shares, the holders of Common Shares are entitled to receive dividends as and when declared by the Board of Directors of the Corporation. In the event of liquidation, dissolution or winding-up of the Corporation, subject to the prior rights of the holders of Preferred Shares, the holders of Common Shares are entitled to receive all the remaining property and assets of the Corporation. The holders of Common Shares are entitled to receive notice of and to attend and to vote at all meetings of the shareholders of the Corporation and each Common Share, when represented at any meeting of the shareholders of the Corporation, carries the right to one vote.
Preferred Shares
The Preferred Shares of the Corporation are issuable from time to time in one or more series as determined by the Board of Directors of the Corporation. The Board of Directors of the Corporation may determine, before issuance, the designation, rights, privileges and restrictions attached to each series of Preferred Shares including the rate of preferential dividends, the dates of payment thereof, the redemption price and the terms of redemption, voting rights and conversion rights (if any), the whole subject to the filing of articles of amendment setting forth the designation, rights, privileges, restrictions, conditions and limitations attaching to the Preferred Shares of such series and the issuance of a certificate of amendment in respect thereof. If any cumulative dividends or amounts payable on return of capital in respect of a series of Preferred Shares are not paid in full, the Preferred Shares of all series shall participate rateably in respect of accumulated dividends and return of capital. The holders of Preferred Shares are entitled to priority over holders of any Common Shares of the Corporation with respect to the payment of dividends or the distribution of assets in the event of liquidation, dissolution or winding-up of the Corporation. Except as required by law or in accordance with any voting rights which may from time to time be attached to any series of Preferred Shares, the holders of the Preferred Shares as a class shall not be entitled to receive notice of, to attend or to vote at any meetings of the shareholders of the Corporation.
| 10 |
DESCRIPTION OF SUBSCRIPTION RECEIPTS
The following description of the terms of Subscription Receipts sets forth certain general terms and provisions of Subscription Receipts in respect of which a Prospectus Supplement may be filed. The particular terms and provisions of Subscription Receipts offered by any Prospectus Supplement, and the extent to which the general terms and provisions described below may apply thereto, will be described in the Prospectus Supplement filed in respect of such Subscription Receipts.
Subscription Receipts may be offered separately or in combination with one or more other Securities. The Subscription Receipts will be issued under a subscription receipt agreement. A copy of the subscription receipt agreement will be filed by the Corporation with the applicable securities commission or similar regulatory authorities after it has been entered into by IMV and will be available electronically at www.sedar.com. Pursuant to the subscription receipt agreement, original purchasers of Subscription Receipts will have a contractual right of rescission against the Corporation, following the issuance of the underlying Common Shares or other securities to such purchasers upon the surrender or deemed surrender of the Subscription Receipts, to receive the amount paid for the Subscription Receipts in the event that this Prospectus and any amendment thereto contains a misrepresentation or is not delivered to such purchaser, provided such remedy for rescission is exercised within 180 days from the closing date of the offering of Subscription Receipts.
The description of general terms and provisions of Subscription Receipts described in any Prospectus Supplement will include, where applicable:
| - | the number of Subscription Receipts offered; |
| - | the price at which the Subscription Receipts will be offered; |
| - | if other than Canadian dollars, the currency or currency unit in which the Subscription Receipts are denominated; |
| - | the procedures for the exchange of the Subscription Receipts into Common Shares or other securities; |
| - | the number of Common Shares or other securities that may be obtained upon exchange of each Subscription Receipt; |
| - | the designation and terms of any other Securities with which the Subscription Receipts will be offered, if any, and the number of Subscription Receipts that will be offered with each Security; |
| - | the terms applicable to the gross proceeds from the sale of the Subscription Receipts plus any interest earned thereon; |
| - | the material tax consequences of owning the Subscription Receipts; and |
| - | any other material terms, conditions and rights (or limitations on such rights) of the Subscription Receipts. |
The Corporation reserves the right to set forth in a Prospectus Supplement specific terms of the Subscription Receipts that are not within the options and parameters set forth in this Prospectus. In addition, to the extent that any particular terms of the Subscription Receipts described in a Prospectus Supplement differ from any of the terms described in this Prospectus, the description of such terms set forth in this Prospectus shall be deemed to have been superseded by the description of such differing terms set forth in such Prospectus Supplement with respect to such Subscription Receipts.
| 11 |
The following description, together with the additional information the Corporation may include in any applicable Prospectus Supplement, summarizes the material terms and provisions of the Warrants that the Corporation may offer under this Prospectus in one or more series. While the terms the Corporation has summarized below will apply generally to any Warrants that it may offer under this Prospectus, the Corporation will describe the particular terms of any series of Warrants that it may offer in more detail in the applicable Prospectus Supplement.
Unless the applicable Prospectus Supplement otherwise indicates, Warrants will be issued under and governed by the terms of one or more warrant indentures (each a “Warrant Indenture”) between the Corporation and a warrant trustee that the Corporation will name in the relevant Prospectus Supplements. Each warrant trustee will be a financial institution organized under the laws of Canada or any province thereof and authorized to carry on business as a trustee.
This summary of some of the provisions of the Warrants is not complete. The statements made in this Prospectus relating to any Warrant Indenture and Warrants to be issued under this Prospectus are summaries of certain anticipated provisions thereof and do not purport to be complete and are subject to, and are qualified in their entirety by reference to, all provisions of the applicable Warrant Indenture or Prospectus Supplement. Prospective purchasers should refer to the Prospectus Supplement and Warrant Indenture, if applicable, relating to the specific Warrants being offered for the complete terms of the Warrants. A copy of any Warrant Indenture relating to an offering of Warrants will be filed by the Corporation with the applicable securities regulatory authorities in Canada after the Corporation has entered into it and will be available electronically at www.sedar.com.
The applicable Prospectus Supplements relating to any Warrants offered by the Corporation will describe the particular terms of those Warrants and include specific terms relating to the offering. This description will include, where applicable:
| - | the designation and aggregate number of Warrants; |
| - | the price at which the Warrants will be offered; |
| - | the date on which the right to exercise the Warrants will commence and the date on which the right will expire; |
| - | the number of securities that may be purchased upon exercise of each Warrant and the price at which and currency or currencies in which the securities may be purchased upon exercise of each Warrant; |
| - | the designation and terms of any Securities with which the Warrants will be offered, if any, and the number of Warrants that will be offered with each Security; |
| - | the date or dates, if any, on or after which the Warrants and the other Securities with which the Warrants will be offered will be transferable separately; |
| - | whether the Warrants will be subject to redemption and, if so, the terms of such redemption provisions; |
| - | whether the Corporation will issue the Warrants as global securities and, if so, the identity of the depositary of the global securities; |
| - | whether the Warrants will be listed on any exchange; |
| - | material United States and Canadian federal income tax consequences of owning the Warrants; and |
| - | any other material terms or conditions of the Warrants. |
The Corporation reserves the right to set forth in a Prospectus Supplement specific terms of the Warrants that are not within the options and parameters set forth in this Prospectus. In addition, to the extent that any particular terms of the Warrants described in a Prospectus Supplement differ from any of the terms described in this Prospectus, the description of such terms set forth in this Prospectus shall be deemed to have been superseded by the description of such differing terms set forth in such Prospectus Supplement with respect to such Warrants.
| 12 |
The Corporation may issue Units comprised of one or more of the other Securities described in this Prospectus in any combination. Each Unit will be issued so that the holder of the Unit is also the holder of each Security included in the Unit. Thus, the holder of a Unit will have the rights and obligations of a holder of each included Security. The unit agreement, if any, under which a Unit is issued may provide that the Securities included in the Unit may not be held or transferred separately, at any time or at any time before a specified date.
The particular terms and provisions of Units offered by any Prospectus Supplement, and the extent to which the general terms and provisions described above may apply thereto, will be described in the Prospectus Supplement filed in respect of such Units.
The following table sets out the details of the issuance by the Corporation of Common Shares, options to purchase Common Shares, warrants to purchase Common Shares, deferred share units, if any, during the 12-month period before the date of this Prospectus:
| Security | Number(1) | Price(1) | Issuance Date | |||||||
| Common Shares(2) | 2,403,846 | $ | 4.16 | June 21, 2017 | ||||||
| Compensation options(3) | 144,231 | $ | 4.22 | June 21, 2017 | ||||||
| Common Shares(4) | 408 | N/A | (5) | June 26, 2017 | ||||||
| Deferred share units(6) | 24,202 | $ | 3.68 | June 30, 2017 | ||||||
| Common Shares(7) | 18,438 | $ | 2.30 | July 10, 2017 | ||||||
| Common Shares(7) | 15,156 | $ | 2.30 | July 19, 2017 | ||||||
| Deferred share units(8) | 25,074 | $ | 3.552 | September 30, 2017 | ||||||
| Common Shares(9) | 73,736 | $ | 1.92 | September 30, 2017 | ||||||
| Common Shares(7) | 8,906 | $ | 2.30 | October 19, 2017 | ||||||
| Common Shares(4) | 3,953 | N/A | (10) | November 15, 2017 | ||||||
| Common Shares(9) | 31,250 | $ | 1.92 | November 24, 2017 | ||||||
| Common Shares(7) | 78,125 | $ | 2.30 | November 24, 2017 | ||||||
| Common Shares(4) | 1,022 | N/A | (11) | December 1, 2017 | ||||||
| Common Shares(9) | 104,986 | $ | 1.92 | December 5, 2017 | ||||||
| Common Shares(7) | 46,875 | $ | 2.30 | December 5, 2017 | ||||||
| Common Shares(7) | 46,875 | $ | 2.30 | December 6, 2017 | ||||||
| Common Shares(7) | 11,250 | $ | 2.30 | December 12, 2017 | ||||||
| Common Shares(4) | 6,323 | N/A | (12) | December 19, 2017 | ||||||
| Deferred share units(13) | 12,101 | $ | 2.30 | December 31, 2017 | ||||||
| Common Shares(4) | 54,852 | N/A | (14) | January 3, 2018 | ||||||
| Common Shares(4) | 9,656 | N/A | (15) | January 8, 2018 | ||||||
| Stock Options(16) | 116,063 | $ | 7.04 | January 16, 2018 | ||||||
| Common Shares(4) | 1,692 | N/A | (5) | January 17, 2018 | ||||||
| Common Shares(4) | 7,631 | N/A | (17) | January 19, 2018 | ||||||
| Stock options(18) | 78,125 | $ | 7.04 | January 22, 2018 | ||||||
| Common Shares(4) | 9,148 | N/A | (19) | January 22, 2018 | ||||||
| Common Shares(20) | 2,246,094 | $ | 6.40 | February 15, 2018 | ||||||
| Common Shares(4) | 190,871 | N/A | (21) | February 26, 2018 | ||||||
| Common Shares(4) | 6,019 | N/A | (22) | February 28, 2018 | ||||||
| Stock Options(31) | 390,625 | $ | 6.40 | March 21, 2018 | ||||||
| Common Shares(4) | 625 | $ | 3.20 | March 22, 2018 | ||||||
| Common Shares(4) | 3,025 | $ | 0.90 | March 22, 2018 | ||||||
| Common Shares(4) | 773 | N/A | (23) | March 26, 2018 | ||||||
| Common Shares(4) | 3,125 | $ | 3.20 | March 27, 2018 | ||||||
| Deferred share units(27) | 15,675 | $ | 6.24 | March 31, 2018 | ||||||
| Stock options(32) | 4,688 | $ | 6.40 | April 1, 2018 | ||||||
| Common Shares(4) | 5,084 | N/A | (24) | April 3, 2018 | ||||||
| 13 |
| Security | Number(1) | Price(1) | Issuance Date | |||||||
| Common Shares(4) | 1,702 | N/A | (25) | April 4, 2018 | ||||||
| Common Shares(4) | 1,972 | N/A | (26) | April 5, 2018 | ||||||
| Common Shares(4) | 10,144 | $ | 0.90 | April 11, 2018 | ||||||
| Common Shares(7) | 22,891 | $ | 2.30 | April 19, 2018 | ||||||
| Common Shares(7) | 17,469 | $ | 2.30 | April 23, 2018 | ||||||
| Common Shares(7) | 15,625 | $ | 2.30 | April 24, 2018 | ||||||
| Common Shares(4) | 1,721 | N/A | (28) | April 27, 2018 | ||||||
| Common Shares(4) | 10,938 | $ | 0.90 | April 30, 2018 | ||||||
| Common Shares(29) | 15,625 | $ | 2.53 | May 1, 2018 | ||||||
| Common Shares(7) | 2,344 | $ | 2.30 | May 1, 2018 | ||||||
| Common Shares(7) | 4,250 | $ | 2.30 | May 3, 2018 | ||||||
| Common Shares(30) | 12,839 | N/A | (30) | May 15, 2018 | ||||||
| Common Shares(7) | 62,500 | $ | 2.30 | May 17, 2018 | ||||||
| Common Shares(7) | 7,812 | $ | 2.30 | May 18, 2018 | ||||||
| Common Shares(7) | 18,467 | $ | 2.30 | May 23, 2018 | ||||||
| Common Shares(4) | 15,625 | $ | 2.53 | May 23, 2018 | ||||||
| Common Shares(4) | 15,625 | $ | 2.11 | May 23, 2018 | ||||||
| Common Shares(4) | 3,125 | $ | 3.20 | May 23, 2018 | ||||||
| Common Shares(4) | 15,625 | $ | 2.37 | May 23, 2018 | ||||||
| Common Shares(4) | 15,625 | $ | 2.11 | May 23, 2018 | ||||||
| Common Shares(7) | 18,467 | $ | 2.30 | May 23, 2018 | ||||||
| Common Shares(7) | 10,812 | $ | 2.30 | May 24, 2018 | ||||||
| Common Shares(7) | 4,250 | $ | 2.30 | May 25, 2018 | ||||||
| Common Shares(7) | 4,109 | $ | 2.30 | May 28, 2018 | ||||||
| Common Shares(7) | 31,250 | $ | 2.30 | May 30, 2018 | ||||||
| Common Shares(7) | 16,328 | $ | 2.30 | May 31, 2018 | ||||||
| Common Shares(7) | 6,250 | $ | 2.30 | June 1, 2018 | ||||||
| (1) | On May 2, 2018, the Corporation filed articles of amendment to give effect to a consolidation of its Common Shares on the basis of 1 post-consolidation Common Share for each 3.2 pre-consolidation Common Shares. Historical information with regards to issuance of Common Shares, options to purchase Common Shares, warrants to purchase Common Shares and Deferred share units (“DSUs”) has been amended to reflect the 3.2 to 1 consolidation. Fractions have been rounded up or down to the nearest whole number and prices have been rounded up or down to the nearest cent. |
| (2) | Common Shares issued pursuant to a bought-deal public offering of Common Shares (the “June 2017 Public Offering”). |
| (3) | Compensation options exercisable at a price of $4.22 per Common Share until June 21, 2019 issued as consideration to the underwriters of the June 2017 Public Offering. |
| (4) | Common Shares issued upon exercise of stock options. |
| (5) | Cashless exercise of 3,125 options. |
| (6) | DSUs issued pursuant to the Corporation’s deferred share unit plan (the “DSU Plan”) at a deemed price of $3.68 per DSU. Each DSU entitles the holder thereof to receive one Common Share on the terms and conditions set forth in the DSU Plan. |
| (7) | Common Shares issued upon exercise of common share purchase warrants (the “2016 Warrants”) being part of units issued pursuant to a bought-deal private placement of units (the “June 2016 Private Placement”). Each 2016 Warrant entitles its holder to purchase one Common Share at a price of $2.30 per Common Share until June 8, 2018. |
| (8) | DSUs issued pursuant to the DSU Plan at a deemed price of $3.55 per DSU. Each DSU entitles the holder thereof to receive one Common Share on the terms and conditions set forth in the DSU Plan. |
| (9) | Common Shares issued upon exercise of non-transferable compensation options exercisable at a price of $1.92 per Common Share until June 8, 2018 issued as a consideration to the underwriters of the June 2016 Private Placement. |
| (10) | Cashless exercise of 7,813 options. |
| (11) | Cashless exercise of 1,885 options. |
| (12) | Cashless exercise of 9,375 options. |
| (13) | DSUs issued pursuant to the DSU Plan at a deemed price of $7.36 per DSU. Each DSU entitles the holder thereof to receive one Common Share on the terms and conditions set forth in the DSU Plan. |
| (14) | Cashless exercise of 78,188 options. |
| (15) | Cashless exercise of 10,938 options. |
| (16) | Grant of stock options governed by the Corporation’s stock option plan exercisable at a price of $7.04 per Common Share until January 16, 2023. |
| (17) | Cashless exercise of 8,738 options. |
| (18) | Grant of stock options governed by the Corporation’s stock option plan exercisable at a price of $7.04 per Common Share until January 22, 2023. |
| (19) | Cashless exercise of 13,438 options. |
| 14 |
| (20) | Common Shares issued pursuant to the February 2018 Public Offering. |
| (21) | Cashless exercise of 293,438 options. |
| (22) | Cashless exercise of 9,750 options. |
| (23) | Cashless exercise of 1,563 options. |
| (24) | Cashless exercise of 7,813 options. |
| (25) | Cashless exercise of 1,992 options. |
| (26) | Cashless exercise of 2,306 options. |
| (27) | DSUs issued pursuant to the DSU Plan at a deemed price of $6.24 per DSU. Each DSU entitles the holder thereof to receive one Common Share on the terms and conditions set forth in the DSU Plan. |
| (28) | Cashless exercise of 2,020 options. |
| (29) | Compensation options exercisable at a price of $2.528 per Common Share until December 9, 2018 issued as consideration to the underwriters of the December 2016 Private Placement. |
| (30) | Common shares issued upon redemption of 26,051 DSUs in accordance with the terms of the DSU Plan. |
| (31) | Grant of stock options governed by the Corporation’s stock option plan exercisable at a price of $6.40 per Common Share until March 21, 2023. |
| (32) | Grant of stock options governed by the Corporation’s stock option plan exercisable at a price of $6.40 per Common Share until April 1, 2023. |
The Common Shares are currently listed on the TSX under the symbol “IMV” and NASDAQ under the symbol “IMV”.
The following table provides the price ranges and trading volume of the Common Shares on the TSX for the periods indicated below:
Price Ranges(1) | Total Cumulative Volume(1) | |||||||||||
| High ($) | Low ($) | |||||||||||
| June 2017 | $ | 4.26 | $ | 3.58 | 429,493 | |||||||
| July 2017 | $ | 4.48 | $ | 3.46 | 621,775 | |||||||
| August 2017 | $ | 3.94 | $ | 3.55 | 370,905 | |||||||
| September 2017 | $ | 3.87 | $ | 3.36 | 528,076 | |||||||
| October 2017 | $ | 4.96 | $ | 3.33 | 1,342,138 | |||||||
| November 2017 | $ | 5.38 | $ | 4.32 | 1,008,102 | |||||||
| December 2017 | $ | 8.16 | $ | 5.06 | 1,514,423 | |||||||
| January 2018 | $ | 8.03 | $ | 5.98 | 1,103,615 | |||||||
| February 2018 | $ | 6.98 | $ | 5.82 | 589,178 | |||||||
| March 2018 | $ | 6.66 | $ | 5.92 | 361,870 | |||||||
| April 2018 | $ | 7.10 | $ | 5.18 | 551,825 | |||||||
| May 2018 | $ | 9.25 | $ | 6.11 | 1,439,544 | |||||||
| June 1-4, 2018 | $ | 9.49 | $ | 8.06 | 199,580 | |||||||
| (1) | On May 2, 2018, the Corporation filed articles of amendment to give effect to a consolidation of its Common Shares on the basis of 1 post-consolidation Common Share for each 3.2 pre-consolidation Common Shares. The post-consolidation Common Shares began trading on TSX on May 10, 2018. Historical trading prices and volumes have been amended to reflect the 3.2 for 1 consolidation. Fractions have been rounded up or down to the nearest whole number and prices have been rounded up or down to the nearest cent. |
| 15 |
An investment in the Corporation’s securities involves risk. Before you invest in the Securities, you should carefully consider the risks contained in or incorporated by reference into this Prospectus and any applicable Prospectus Supplement, including the risks described below and in the AIF and Annual MD&A, which are incorporated by reference into this Prospectus. The discussion of risks related to the business of the Corporation contained in or incorporated by reference into this Prospectus comprises material risks of which the Corporation is aware. If any of the events or developments described actually occurs, the business, financial condition or results of operations of the Corporation would likely be adversely affected.
Risks Relating to the Securities
The share price has been and is likely to continue to be volatile and an investment in Common Shares may suffer a decline in value.
You should consider an investment in Common Shares as risky and invest only if you can withstand a significant loss and wide fluctuations in the market value of your investment. The Corporation receives only limited attention by securities analysts and frequently experience an imbalance between supply and demand for Common Shares. The market price of the Common Shares has been highly volatile and is likely to continue to be volatile. This leads to a heightened risk of securities litigation pertaining to such volatility. Factors such as the financial position of the Corporation and the ability of the Corporation to continue as a going concern; the ability to raise additional capital; the progress of the clinical trials; the ability to obtain partners and collaborators to assist with the future development of the products; general market conditions; announcements of technological innovations or new product candidates by the Corporation, the Corporation collaborators or its competitors; published reports by securities analysts; developments in patent or other intellectual property rights; public concern as to the safety and efficacy of drugs that the Corporation and its competitors develop; and shareholder interest in the Common Shares all contribute to the volatility of the share price.
Future sales of Common Shares by the Corporation or by its existing shareholders could cause share price to fall.
The issuance of Common Shares by the Corporation could result in significant dilution in the equity interest of existing shareholders and adversely affect the market price of the Common Shares. Sales by existing shareholders of a large number of Common Shares in the public market and the issuance of Common Shares issued in connection with strategic alliances, or the perception that such additional sales could occur, could cause the market price of the Common Shares to decline and have an undesirable impact on the Corporation’s ability to raise capital.
Dilution of purchasers.
Purchasers who purchase Securities offered pursuant to this Prospectus may pay more for the Common Shares than the amounts paid by existing shareholders or security holders of the Corporation for their Common Shares. As a result, such purchasers may incur immediate and substantial dilution. Convertible securities have been issued and may be issued in the future by the Corporation at a lower price than the current market value of the Common Shares, consequently, purchasers who purchase Common Shares under the offering of Securities hereunder may incur substantial dilution in the near future.
No dividends have been paid on the Common Shares.
The Corporation has paid no cash dividends on any of its Common Shares to date and currently intends to retain its future earnings, if any, to fund the development growth of its businesses. In addition, the terms of any future debt or credit facility may preclude the Corporation from paying any dividends unless certain consents are obtained and certain conditions are met.
United States investors may not be able to obtain enforcement of civil liabilities against the Corporation.
The enforcement by investors of civil liabilities under the United States federal or state securities laws may be affected adversely by the fact that the Corporation is governed by the Canada Business Corporations Act, that the majority of the Corporation officers and directors are residents of Canada, and that all, or a substantial portion of their assets and a substantial portion of the Corporation assets, are located outside the United States. It may not be possible for investors to effect service of process within the United States on certain of its directors and officers or enforce judgments obtained in the United States courts against the Corporation or certain of the Corporation directors and officers based upon the civil liability provisions of United States federal securities laws or the securities laws of any state of the United States.
| 16 |
There is some doubt as to whether a judgment of a United States court based solely upon the civil liability provisions of United States federal or state securities laws would be enforceable in Canada against the Corporation or its directors and officers. There is also doubt as to whether an original action could be brought in Canada against the Corporation or its directors and officers to enforce liabilities based solely upon United States federal or state securities laws.
If the Corporation is characterized as a passive foreign investment company, U.S. holders may be subject to adverse U.S. federal income tax consequences.
U.S. investors should be aware that they could be subject to certain adverse U.S. federal income tax consequences in the event that the Corporation is classified as a “passive foreign investment company” (“PFIC”) for U.S. federal income tax purposes. The determination of whether the Corporation is a PFIC for a taxable year depends, in part, on the application of complex U.S. federal income tax rules, which are subject to differing interpretations, and the determination will depend on the composition of the Corporation’s income, expenses and assets from time to time and the nature of the activities performed by the Corporation’s officers and employees. The Corporation may be a PFIC in one or more prior tax years, in the current tax year and in subsequent tax years. Prospective investors should carefully read the tax discussion in any applicable Prospectus Supplement for more information and consult their own tax advisers regarding the likelihood and consequences of the Corporation being treated as a PFIC for U.S. federal income tax purposes, including the advisability of making certain elections that may mitigate certain possible adverse U.S. federal income tax consequences that may result in an inclusion in gross income without receipt of such income.
As a foreign private issuer, the Corporation is subject to different U.S. securities laws and rules than a domestic U.S. issuer, which may limit the information publicly available to its U.S. shareholders.
The Corporation is a foreign private issuer under applicable U.S. federal securities laws and, therefore, is not required to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act and related rules and regulations. As a result, the Corporation does not file the same reports that a U.S. domestic issuer would file with the SEC, although it will be required to file with or furnish to the SEC the continuous disclosure documents that the Corporation is required to file in Canada under Canadian securities laws. In addition, the Corporation’s officers, directors and principal shareholders are exempt from the reporting and “short swing” profit recovery provisions of Section 16 of the Exchange Act. Therefore, the Corporation’s shareholders may not know on as timely a basis when its officers, directors and principal shareholders purchase or sell securities of IMV as the reporting periods under the corresponding Canadian insider reporting requirements are longer. In addition, as a foreign private issuer, the Corporation is exempt from the proxy rules under the Exchange Act.
The Corporation may lose its foreign private issuer status in the future, which could result in significant additional costs and expenses to the Corporation.
In order to maintain its current status as a foreign private issuer, a majority of the Corporation’s Common Shares must be either directly or indirectly owned of record by non-residents of the United States unless the Corporation also satisfies one of the additional requirements necessary to preserve this status. The Corporation may in the future lose its foreign private issuer status if a majority of the Common Shares are owned of record in the United States and the Corporation fails to meet the additional requirements necessary to avoid loss of foreign private issuer status. The regulatory and compliance costs to the Corporation under U.S. federal securities laws as a U.S. domestic issuer may be significantly more than the costs the Corporation incurs as a Canadian foreign private issuer eligible to use MJDS. If the Corporation is not a foreign private issuer, it would not be eligible to use the MJDS or other foreign issuer forms and would be required to file periodic and current reports and registration statements on U.S. domestic issuer forms with the SEC, which are more detailed and extensive than the forms available to a foreign private issuer. In addition, the Corporation may lose the ability to rely upon exemptions from NASDAQ corporate governance requirements that are available to foreign private issuers.
| 17 |
CERTAIN CANADIAN FEDERAL INCOME TAX CONSiderations
The applicable Prospectus Supplement will describe certain Canadian federal income tax consequences to an investor who is a non-resident of Canada of acquiring any Securities offered thereunder, including whether the payments of distributions on the Securities will be subject to Canadian non-resident withholding tax.
CERTAIN U.S. FEDERAL INCOME TAX CONSIDERATIONS
The following is a general summary of certain material U.S. federal income tax considerations relevant to a U.S. Holder (as defined below) arising from and relating to the acquisition, ownership, and disposition of Common Shares acquired pursuant to this Prospectus.
This summary is for general information purposes only and does not purport to be a complete analysis or listing of all potential U.S. federal income tax consequences that may apply to a U.S. Holder arising from and relating to the acquisition, ownership, and disposition of Common Shares. In addition, this summary does not take into account the individual facts and circumstances of any particular U.S. Holder that may affect the U.S. federal income tax consequences to such U.S. Holder. Accordingly, this summary is not intended to be, and should not be construed as, legal or U.S. federal income tax advice with respect to any U.S. Holder. Except as discussed below, this summary does not discuss applicable income tax reporting requirements. This summary does not address the U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and non U.S. tax consequences to U.S. Holders of the acquisition, ownership, and disposition of Common Shares. Each prospective U.S. Holder should consult its own tax advisor regarding the U.S. federal, U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences relating to the acquisition, ownership, and disposition of Common Shares.
No legal opinion from U.S. legal counsel or ruling from the Internal Revenue Service (the “IRS”) has been requested, or will be obtained, regarding the U.S. federal income tax consequences of the acquisition, ownership, and disposition of Common Shares. This summary is not binding on the IRS, and the IRS is not precluded from taking a position that is different from, and contrary to, the positions taken in this summary. In addition, because the authorities upon which this summary is based are subject to various interpretations, the IRS and the U.S. courts could disagree with one or more of the positions taken in this summary.
Scope of this Summary
Authorities
This summary is based on the Internal Revenue Code of 1986, as amended (the “Code”), Treasury Regulations (whether final, temporary, or proposed), published rulings of the IRS, published administrative positions of the IRS, the Convention Between Canada and the United States of America with Respect to Taxes on Income and on Capital, signed September 26, 1980, as amended (the “Canada-U.S. Tax Convention”), and U.S. court decisions that are applicable and, in each case, as in effect and available, as of the date of this document. Any of the authorities on which this summary is based could be changed in a material and adverse manner at any time, and any such change could be applied on a retroactive or prospective basis. This summary does not discuss the potential effects, whether adverse or beneficial, of any proposed legislation that, if enacted, could be applied on a retroactive or prospective basis.
U.S. Holders
For purposes of this summary, the term “U.S. Holder” means a beneficial owner of Common Shares acquired pursuant to this Prospectus that is for U.S. federal income tax purposes:
| - | an individual who is a citizen or resident of the U.S.; |
| - | a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) organized under the laws of the U.S., any state thereof or the District of Columbia; |
| - | an estate whose income is subject to U.S. federal income taxation regardless of its source; or |
| 18 |
| - | a trust that (a) is subject to the primary supervision of a court within the U.S. and one or more U.S. persons has the authority to make all substantial decisions of the trust or (b) has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person. |
Non-U.S. Holders
For purposes of this summary, a “non-U.S. Holder” is a beneficial owner of Common Shares that is not a U.S. Holder and is not a partnership for U.S. federal income tax purposes. This summary does not address the U.S. federal income tax consequences to non-U.S. Holders arising from and relating to the acquisition, ownership, and disposition of Common Shares. Accordingly, a non-U.S. Holder should consult its own tax advisor regarding the U.S. federal, U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and foreign tax consequences (including the potential application of and operation of any income tax treaties) relating to the acquisition, ownership, and disposition of Common Shares.
U.S. Holders Subject to Special U.S. Federal Income Tax Rules Not Addressed
This summary does not address the U.S. federal income tax considerations applicable to U.S. Holders that are subject to special provisions under the Code, including, but not limited to, the following: (a) U.S. Holders that are tax-exempt organizations, qualified retirement plans, individual retirement accounts, or other tax-deferred accounts; (b) U.S. Holders that are financial institutions, underwriters, insurance companies, real estate investment trusts, or regulated investment companies; (c) U.S. Holders that are broker-dealers, dealers, or traders in securities or currencies that elect to apply a mark-to-market accounting method; (d) U.S. Holders that have a “functional currency” other than the U.S. dollar; (e) U.S. Holders that own Common Shares as part of a straddle, hedging transaction, conversion transaction, constructive sale, or other arrangement involving more than one position; (f) U.S. Holders that acquired Common Shares in connection with the exercise of employee stock options or otherwise as compensation for services; (g) U.S. Holders that hold Common Shares other than as a capital asset within the meaning of Section 1221 of the Code (generally, property held for investment purposes); or (h) U.S. Holders that own, have owned or will own (directly, indirectly, or by attribution) 10% or more of the total combined voting power of the outstanding Common Shares of the Corporation. This summary also does not address the U.S. federal income tax considerations applicable to U.S. Holders who are: (a) U.S. expatriates or former long-term residents of the U.S.; (b) persons that have been, are, or will be a resident or deemed to be a resident in Canada for purposes of the Tax Act; (c) persons that use or hold, will use or hold, or that are or will be deemed to use or hold Common Shares in connection with carrying on a business in Canada; (d) persons whose Common Shares constitute “taxable Canadian property” under the Tax Act; or (e) persons that have a permanent establishment in Canada for the purposes of the Canada-U.S. Tax Convention. U.S. Holders that are subject to special provisions under the Code, including, but not limited to, U.S. Holders described immediately above, should consult their own tax advisor regarding the U.S. federal, U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences relating to the acquisition, ownership and disposition of Common Shares.
If an entity or arrangement that is classified as a partnership for U.S. federal income tax purposes holds Common Shares, the U.S. federal income tax consequences to such entity and the partners (or other owners) of such partnership generally will depend on the activities of the partnership and the status of such partners. This summary does not address the tax consequences to any such owner. Partners of entities or arrangements that are classified as partnerships for U.S. federal income tax purposes should consult their own tax advisors regarding the U.S. federal income tax consequences arising from and relating to the acquisition, ownership, and disposition of Common Shares.
Ownership and Disposition of Common Shares
The following discussion is subject in its entirety to the rules described below under the heading “Passive Foreign Investment Company Rules”.
| 19 |
Taxation of Distributions
A U.S. Holder that receives a distribution, including a constructive distribution, with respect to a Common Share will be required to include the amount of such distribution in gross income as a dividend (without reduction for any Canadian or foreign income tax withheld from such distribution) to the extent of the current or accumulated “earnings and profits” of the Corporation, as computed for U.S. federal income tax purposes. To the extent that a distribution exceeds the current and accumulated “earnings and profits” of the Corporation, such distribution will be treated first as a tax-free return of capital to the extent of a U.S. Holder’s tax basis in the Common Shares and thereafter as gain from the sale or exchange of such Common Shares (see “Sale or Other Taxable Disposition of Common Shares”). However, the Corporation may not maintain the calculations of earnings and profits in accordance with U.S. federal income tax principles, and each U.S. Holder should therefore assume that any distribution by the Corporation with respect to the Common Shares will constitute ordinary dividend income. Dividends received on Common Shares generally will not be eligible for the “dividends received deduction” available to U.S. corporate shareholders receiving dividends from U.S. corporations.
Subject to applicable limitations and provided the Corporation is eligible for the benefits of the Canada – U.S. Tax Convention or the Common Shares are readily tradable on a United States securities market, dividends paid by the Corporation to non-corporate U.S. Holders, including individuals, generally will be eligible for the preferential tax rates applicable to long-term capital gains for dividends provided certain holding period and other conditions are satisfied, including that the Corporation not be classified as a PFIC in the tax year of distribution or in the preceding tax year. The dividend rules are complex, and each U.S. Holder should consult its own tax advisor regarding the application of such rules.
Sale or Other Taxable Disposition of Common Shares
A U.S. Holder will recognize gain or loss on the sale or other taxable disposition of Common Shares in an amount equal to the difference, if any, between (a) the amount of cash plus the fair market value of any property received and (b) such U.S. Holder’s tax basis in such Common Shares sold or otherwise disposed of. Any such gain or loss generally will be capital gain or loss, which will be long-term capital gain or loss if, at the time of the sale or other disposition, such Common Shares are held for more than one year.
Preferential tax rates apply to long-term capital gains of non-corporate U.S. Holders. There are currently no preferential tax rates for long-term capital gains of a U.S. Holder that is a corporation. Deductions for capital losses are subject to significant limitations under the Code. A U.S. Holder’s tax basis in Common Shares generally will be such U.S. Holder’s U.S. dollar cost for such Common Shares.
PFIC Status of the Corporation
If the Corporation is or becomes a PFIC, the preceding sections of this summary may not describe the U.S. federal income tax consequences to U.S. Holders of the ownership and disposition of Common Shares. The U.S. federal income tax consequences of owning and disposing of Common Shares if the Corporation is or becomes a PFIC are described below under the heading “Tax Consequences if the Corporation is a PFIC”.
A non-U.S. corporation is a PFIC for each tax year in which (i) 75% or more of its gross income is passive income (as defined for U.S. federal income tax purposes) (the “income test”) or (ii) on average for such tax year, 50% or more (by value) of its assets either produces or is held for the production of passive income (the “asset test”). For purposes of the PFIC provisions, “gross income” generally includes sales revenues less cost of goods sold, plus income from investments and from incidental or outside operations or sources, and “passive income” generally includes dividends, interest, certain rents and royalties, and certain gains from commodities or securities transactions. In determining whether or not it is a PFIC, a non-U.S. corporation is required to take into account its pro rata portion of the income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value).
Under certain attribution and indirect ownership rules, if the Corporation is a PFIC, U.S. Holders will generally be deemed to own their proportionate shares of the Corporation’s direct or indirect equity interests in any company that is also a PFIC (a “Subsidiary PFIC”), and will be subject to U.S. federal income tax on their proportionate share of (a) any “excess distributions”, as described below, on the stock of a Subsidiary PFIC and (b) a disposition or deemed disposition of the stock of a Subsidiary PFIC by the Corporation or another Subsidiary PFIC, both as if such U.S. Holders directly held the shares of such Subsidiary PFIC. In addition, U.S. Holders may be subject to U.S. federal income tax on any indirect gain realized on the stock of a Subsidiary PFIC on the sale or disposition of Common Shares. Accordingly, U.S. Holders should be aware that they could be subject to tax even if no distributions are received and no redemptions or other dispositions of the Corporation’s Common Shares are made.
| 20 |
The determination of PFIC status is inherently factual, is subject to a number of uncertainties, and can be determined only annually at the close of the tax year in question. Additionally, the analysis depends, in part, on the application of complex U.S. federal income tax rules, which are subject to differing interpretations. There can be no assurance that the Corporation will or will not be determined to be a PFIC for the current tax year or any prior or future tax year, and no opinion of legal counsel or ruling from the IRS concerning the status of the Corporation as a PFIC has been obtained or will be requested. U.S. Holders should consult their own U.S. tax advisors regarding the PFIC status of the Corporation.
Tax Consequences if the Corporation is a PFIC
If the Corporation is a PFIC for any tax year during which a U.S. Holder holds Common Shares, special rules may increase such U.S. Holder’s U.S. federal income tax liability with respect to the ownership and disposition of such shares. If the Corporation meets the income test or the asset test for any tax year during which a U.S. Holder owns Common Shares, the Corporation will be treated as a PFIC with respect to such U.S. Holder for that tax year and for all subsequent tax years, regardless of whether the Corporation meets the income test or the asset test for such subsequent tax years, unless the U.S. Holder elects to recognize any unrealized gain in the Common Shares or makes a timely and effective QEF Election or Mark-to-Market Election.
Under the default PFIC rules:
| - | any gain realized on the sale or other disposition (including dispositions and certain other events that would not otherwise be treated as taxable events) of Common Shares (including an indirect disposition of the stock of any Subsidiary PFIC) and any “excess distribution” (defined as a distribution to the extent it (together with all other distributions received in the relevant tax year) exceeds 125% of the average annual distributions received during the preceding three years) received on Common Shares or with respect to the stock of a Subsidiary PFIC will be allocated ratably to each day of such U.S. Holder’s holding period for the Common Shares; |
| - | the amount allocated to the current tax year and any year prior to the first year in which the Corporation was a PFIC will be taxed as ordinary income in the current year; the amount allocated to each of the other tax years (the “Prior PFIC Years”) will be subject to tax at the highest ordinary income tax rate in effect for the applicable class of taxpayer for that year; and |
| - | an interest charge will be imposed with respect to the resulting tax attributable to each Prior PFIC Year, which interest charge is not deductible by non-corporate U.S. Holders. |
A U.S. Holder that makes a timely and effective “mark-to-market” election under Section 1296 of the Code (a “Mark-to-Market Election”) or a timely and effective election to treat the Corporation and each Subsidiary PFIC as a “qualified electing fund” (a “QEF”) under Section 1295 of the Code (a “QEF Election”) may generally mitigate or avoid the PFIC consequences described above with respect to Common Shares.
U.S. Holders should be aware that, for each tax year, if any, that the Corporation is a PFIC, the Corporation can provide no assurances that it will satisfy the record keeping requirements of a PFIC, or that it will make available to U.S. Holders the information such U.S. Holders require to make a QEF Election with respect to the Corporation or any Subsidiary PFIC. U.S. Holders are urged to consult their own tax advisors regarding the potential application of the PFIC rules to the ownership and disposition of Common Shares, and the availability of certain U.S. tax elections under the PFIC rules.
A timely and effective QEF Election requires a U.S. Holder to include currently in gross income each year its pro rata share of the Corporation’s ordinary earnings and net capital gains, regardless of whether such earnings and gains are actually distributed. Thus, a U.S. Holder could have a tax liability with respect to such ordinary earnings or gains without a corresponding receipt of cash from the Corporation. If the Corporation is a QEF with respect to a U.S. Holder, the U.S. Holder’s basis in the Common Shares will be increased to reflect the amount of the taxed but undistributed income. Distributions of income that had previously been taxed will result in a corresponding reduction of basis in the Common Shares and will not be taxed again as a distribution to a U.S. Holder. Taxable gains on the disposition of Common Shares by a U.S. Holder that has made a timely and effective QEF Election are generally capital gains. A U.S. Holder must make a QEF Election for the Corporation and each Subsidiary PFIC if it wishes to have this treatment. To make a QEF Election, a U.S. Holder will need to have an annual information statement from the Corporation setting forth the ordinary earnings and net capital gains for the year. In general, a U.S. Holder must make a QEF Election on or before the due date for filing its income tax return for the first year to which the QEF Election will apply. Under applicable Treasury Regulations, a U.S. Holder will be permitted to make retroactive elections in particular circumstances, including if it had a reasonable belief that the Corporation was not a PFIC and filed a protective statement. If a U.S. Holder owns PFIC stock indirectly through another PFIC, separate QEF Elections must be made for the PFIC in which the U.S. Holder is a direct shareholder and the Subsidiary PFIC for the QEF rules to apply to both PFICs. Each U.S. Holder should consult its own tax advisor regarding the availability and desirability of, and procedure for, making a timely and effective QEF Election for the Corporation and any Subsidiary PFIC.
| 21 |
A Mark-to-Market Election may be made with respect to stock in a PFIC if such stock is “regularly traded” on a “qualified exchange or other market” (within the meaning of the Code and the applicable Treasury Regulations). A class of stock that is traded on one or more qualified exchanges or other markets is considered to be “regularly traded” for any calendar year during which such class of stock is traded in other than de minimis quantities on at least 15 days during each calendar quarter. If the Common Shares are considered to be “regularly traded” within this meaning, then a U.S. Holder generally will be eligible to make a Mark-to-Market Election with respect to its shares. However, there is no assurance that the Common Shares will be or remain “regularly traded” for this purpose. A Mark-to-Market Election may not be made with respect to the stock of any Subsidiary PFIC because such stock is not marketable. Hence, a Mark-to-Market Election will not be effective to eliminate the application of the default rules of Section 1291 of the Code, described above, with respect to deemed dispositions of Subsidiary PFIC stock or excess distributions with respect to a Subsidiary PFIC. A U.S. Holder that makes a timely and effective Mark-to-Market Election with respect to Common Shares generally will be required to recognize as ordinary income in each tax year in which the Corporation is a PFIC an amount equal to the excess, if any, of the fair market value of such shares as of the close of such taxable year over the U.S. Holder’s adjusted tax basis in such shares as of the close of such taxable year. A U.S. Holder’s adjusted tax basis in the Common Shares generally will be increased by the amount of ordinary income recognized with respect to such shares. If the U.S. Holder’s adjusted tax basis in the Common Shares as of the close of a tax year exceeds the fair market value of such shares as of the close of such taxable year, the U.S. Holder generally will recognize an ordinary loss, but only to the extent of net mark-to-market income recognized with respect to such shares for all prior taxable years. A U.S. Holder’s adjusted tax basis in its Common Shares generally will be decreased by the amount of ordinary loss recognized with respect to such shares. Any gain recognized upon a disposition of the Common Shares generally will be treated as ordinary income, and any loss recognized upon a disposition generally will be treated as an ordinary loss to the extent of net mark-to-market income recognized for all prior taxable years. Any loss recognized in excess thereof will be taxed as a capital loss. Capital losses are subject to significant limitations under the Code.
Each U.S. Holder should consult its own tax advisor regarding the availability and desirability of, and procedure for, making a timely and effective Mark-to-Market Election with respect to the Common Shares.
Additional Considerations
Additional Tax on Passive Income
Individuals, estates and certain trusts whose income exceeds certain thresholds will be required to pay a 3.8% Medicare surtax on “net investment income” including, among other things, dividends and net gain from disposition of property (other than property held in certain trades or businesses). Special rules apply to PFICs. U.S. Holders should consult with their own tax advisors regarding the effect, if any, of this tax on their ownership and disposition of Common Shares.
Receipt of Foreign Currency
The amount of any distribution paid in Canadian dollars to a U.S. Holder in connection with the ownership of the Common Shares, or on the sale, exchange or other taxable disposition of Common Shares, will be included in the gross income of a U.S. Holder as translated into U.S. dollars calculated by reference to the exchange rate prevailing on the date of actual or constructive receipt of the payment, regardless of whether the Canadian dollars are converted into U.S. dollars at that time. If the Canadian dollars received are not converted into U.S. dollars on the date of receipt, a U.S. Holder will have a basis in the Canadian dollars equal to their U.S. dollar value on the date of receipt. Any U.S. Holder who receives payment in Canadian dollars and engages in a subsequent conversion or other disposition of the Canadian dollars may have a foreign currency exchange gain or loss that would be treated as ordinary income or loss, and generally will be U.S. source income or loss for foreign tax credit purposes. Different rules apply to U.S. Holders who use the accrual method with respect to foreign currency. Each U.S. Holder should consult its own U.S. tax advisor regarding the U.S. federal income tax consequences of receiving, owning, and disposing of Canadian dollars.
| 22 |
Foreign Tax Credit
Subject to the PFIC rules discussed above, a U.S. Holder that pays (whether directly or through withholding) Canadian income tax with respect to dividends paid on the Common Shares generally will be entitled, at the election of such U.S. Holder, to receive either a deduction or a credit for such Canadian income tax paid. Generally, a credit will reduce a U.S. Holder’s U.S. federal income tax liability on a dollar-for-dollar basis, whereas a deduction will reduce a U.S. Holder’s income subject to U.S. federal income tax. This election is made on a year-by-year basis and applies to all foreign taxes paid (whether directly or through withholding) by a U.S. Holder during a year. Complex limitations apply to the foreign tax credit, including the general limitation that the credit cannot exceed the proportionate share of a U.S. Holder’s U.S. federal income tax liability that such U.S. Holder’s “foreign source” taxable income bears to such U.S. Holder’s worldwide taxable income. In applying this limitation, a U.S. Holder’s various items of income and deduction must be classified, under complex rules, as either “foreign source” or “U.S. source”. Generally, dividends paid by a foreign corporation should be treated as foreign source for this purpose, and gains recognized on the sale of stock of a foreign corporation by a U.S. Holder should be treated as U.S. source for this purpose, except as otherwise provided in an applicable income tax treaty, and if an election is properly made under the Code. However, the amount of a distribution with respect to the Common Shares that is treated as a “dividend” may be lower for U.S. federal income tax purposes than it is for Canadian federal income tax purposes, resulting in a reduced foreign tax credit allowance to a U.S. Holder. In addition, this limitation is calculated separately with respect to specific categories of income. The foreign tax credit rules are complex, and each U.S. Holder should consult its own U.S. tax advisor regarding the foreign tax credit rules.
Special rules apply to the amount of foreign tax credit that a U.S. Holder may claim on a distribution from a PFIC. Subject to such special rules, non-U.S. taxes paid with respect to any distribution in respect of stock in a PFIC are generally eligible for the foreign tax credit. The rules relating to distributions by a PFIC and their eligibility for the foreign tax credit are complex, and a U.S. Holder should consult its own tax advisor regarding their application to the U.S. Holder.
Backup Withholding and Information Reporting
Under U.S. federal income tax law and Treasury Regulations, certain categories of U.S. Holders must file information returns with respect to their investment in, or involvement in, a non-U.S. corporation. For example, U.S. return disclosure obligations (and related penalties) are imposed on U.S. Holders that hold certain specified foreign financial assets in excess of certain threshold amounts. The definition of “specified foreign financial assets” includes not only financial accounts maintained in foreign financial institutions, but also, if held for investment and not in an account maintained by certain financial institutions, any stock or security issued by a non-U.S. person, any financial instrument or contract held for investment that has an issuer or counterparty other than a U.S. person and any interest in a foreign entity. U.S. Holders may be subject to these reporting requirements unless their Common Shares are held in an account at certain financial institutions. Penalties for failure to file certain of these information returns are substantial. U.S. Holders should consult with their own tax advisors regarding the requirements of filing information returns on IRS Form 8938, and, if applicable, filing obligations relating to the PFIC rules, including possible reporting on IRS Form 8621.
Payments made within the U.S. or by a U.S. payor or U.S. middleman of (a) distributions on the Common Shares, and (b) proceeds arising from the sale or other taxable disposition of Common Shares generally will be subject to information reporting. In addition, backup withholding, currently at a rate of 24% for the 2018 to 2025 tax years (increasing to 28% for tax years after 2025), may apply to such payments if a U.S. Holder (a) fails to furnish such U.S. Holder’s correct U.S. taxpayer identification number (generally on IRS Form W-9), (b) furnishes an incorrect U.S. taxpayer identification number, (c) is notified by the IRS that such U.S. Holder has previously failed to properly report items subject to backup withholding, or (d) fails to certify, under penalty of perjury, that such U.S. Holder has furnished its correct U.S. taxpayer identification number and that the IRS has not notified such U.S. Holder that it is subject to backup withholding. Certain exempt persons generally are excluded from these information reporting and backup withholding rules. Backup withholding is not an additional tax. Any amounts withheld under the U.S. backup withholding rules will be allowed as a credit against a U.S. Holder’s U.S. federal income tax liability, if any, or will be refunded, if such U.S. Holder furnishes required information to the IRS in a timely manner. The information reporting and backup withholding rules may apply even if, under the Canada-U.S. Tax Convention, payments are exempt from the dividend withholding tax or otherwise eligible for a reduced withholding rate. This discussion of reporting requirements set forth above is not intended to constitute an exhaustive description of all reporting requirements that may apply to a U.S. Holder. A failure to satisfy certain reporting requirements may result in an extension of the time period during which the IRS can assess a tax, and under certain circumstances, such an extension may apply to assessments of amounts unrelated to any unsatisfied reporting requirements. Each U.S. Holder should consult its own tax advisor regarding the information reporting and backup withholding rules.
| 23 |
THE ABOVE SUMMARY IS NOT INTENDED TO CONSTITUTE A COMPLETE ANALYSIS OF ALL U.S. TAX CONSIDERATIONS APPLICABLE TO U.S. HOLDERS WITH RESPECT TO THE OWNERSHIP AND DISPOSITION OF COMMON SHARES. U.S. HOLDERS SHOULD CONSULT THEIR OWN TAX ADVISORS AS TO THE TAX CONSIDERATIONS APPLICABLE TO THEM IN THEIR PARTICULAR CIRCUMSTANCES.
Unless specified in the applicable Prospectus Supplement, certain Canadian legal matters will be passed upon on behalf of the Corporation by McCarthy Tétrault LLP. Certain legal matters relating to United States law will be passed upon on behalf of the Corporation by Troutman Sanders LLP. As of the date hereof, the partners and associates of McCarthy Tétrault LLP, as a group, and the partners and associates of Troutman Sanders LLP, as a group, beneficially own, directly or indirectly, less than 1% of the outstanding Common Shares.
Any Securities offered pursuant to this Prospectus, including by way of at-the-market offerings, will be conducted in accordance with applicable securities legislation in Canada and the United States, and, if applicable, will be subject to regulatory approval or exemptive relief.
AUDITOR, TRANSFER AGENT AND REGISTRAR
The auditor of the Corporation is PricewaterhouseCoopers LLP, Chartered Professional Accountants, Halifax, Nova Scotia, Canada.
The transfer agent and registrar for the Common Shares is Computershare Investor Services Inc., at its principal offices located in Toronto, Ontario, Canada or Montréal, Québec, Canada.
Albert Scardino and Wayne Pisano, directors of the Corporation, both reside outside of Canada and have appointed IMV Inc., #53-1344 Summer Street, Suite 412, Halifax, Nova Scotia, Canada, BH3 0A8, as agent for service of process.
Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process.
STATUTORY RIGHTS OF WITHDRAWAL AND RESCISSION
Securities legislation in certain of the provinces of Canada provides purchasers with the right to withdraw from an agreement to purchase securities. This right may be exercised within two business days after receipt or deemed receipt of a short form prospectus and any amendment. In several of the provinces of Canada, the securities legislation further provides a purchaser with remedies for rescission or, in some jurisdictions, revisions of the price or damages if the short form prospectus and any amendment contains a misrepresentation or is not delivered to the purchaser, provided that the remedies for rescission, revision of the price or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province for the particulars of these rights or consult with a legal advisor.
| 24 |
In an offering of Preferred Shares, Subscription Receipts, Warrants and Units (collectively, “Convertible Securities”), investors are cautioned that the statutory right of action for damages for a misrepresentation contained in the Prospectus and the accompanying Prospectus Supplements is limited, in certain provincial securities legislation, to the price at which such security is offered to the public under the prospectus offering. This means that, under the securities legislation of certain provinces, if the purchaser pays additional amounts upon conversion, exchange or exercise of the security, those amounts may not be recoverable under the statutory right of action for damages that applies in those provinces. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province for the particulars of this right of action for damages or consult with a legal advisor. By virtue of their purchase of Convertible Securities, original purchasers will have a contractual right of rescission against the Corporation in respect of the conversion, exchange or exercise of such Convertible Securities. The contractual right of rescission will entitle such original purchasers to receive the amount paid upon conversion, exchange or exercise, upon surrender of the securities issued to such purchaser upon conversion of such Convertible Securities, in the event that this Prospectus, as supplemented by an applicable Prospectus Supplement relating to such Convertible Securities, as amended, contains a misrepresentation, provided that the right of rescission is exercised within 180 days of the date of the purchase of the Convertible Securities. This contractual right of rescission will be consistent with the statutory right of rescission described under section 137 of the Securities Act (Nova Scotia), and is in addition to any other right or remedy available to original purchasers under section 137 the Securities Act (Nova Scotia) or otherwise to law. The purchaser should refer to any applicable provisions of the securities legislation of the province in which the purchaser resides for the particulars of these rights, or consult with a legal advisor.
The Corporation is incorporated under, and governed by, the laws of Canada. Many of its officers and directors and experts named in this Prospectus are resident outside of the United States, and a majority of their assets, and the assets of IMV, are located outside the United States. As a result, it may be difficult for U.S. investors to effect service of process within the United States upon those directors, officers or experts who are not residents of the United States, or to realize in the United States upon judgments of courts of the United States predicated upon civil liability of such directors, officers or experts under U.S. federal securities laws.
IMV has filed with the SEC, concurrently with the filing of its U.S. Registration Statement on Form F-10 of which this Prospectus forms a part, an appointment of agent for service of process on Form F-X. Under the Form F-X, IMV appointed C T Corporation System as its agent for service of process in the United States in connection with any investigation or administrative proceeding conducted by the SEC, and any civil suit or action brought against or involving IMV in a U.S. court arising out of or related to or concerning the offering of Securities under the U.S. Registration Statement. However, it may not be possible for investors to enforce outside the United States judgments against IMV obtained in the United States in any such actions, including actions predicated upon the civil liability provisions of the United States federal and state securities laws.
| 25 |
CERTIFICATE OF THE CORPORATION
Dated: June 5, 2018
This short form base shelf prospectus, together with the documents incorporated by reference, constitutes full, true and plain disclosure of all material facts relating to the securities offered in this prospectus as required by the securities legislation of British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Québec, Nova Scotia and Newfoundland and Labrador.
| /s/ Frederic Ors | /s/ Pierre Labbé | ||||
| Frederic
Ors Chief Executive Officer |
Pierre
Labbé Chief Financial Officer | ||||
| On behalf of the Board of Directors | |||||
| /s/ James Hall | /s/ Shermaine Tilley | ||||
| James
Hall Director |
Shermaine
Tilley Director | ||||
| C-1 |
Common Shares

IMV INC.
Prospectus Supplement
, 2019
| Wells Fargo Securities | Raymond James | |
| B. Riley FBR |