
No securities regulatory authority has expressed an opinion about these securities and it is an offence to claim otherwise. This prospectus supplement, together with the short form base shelf prospectus dated June 5, 2018 to which it relates, as amended or supplemented, and each document incorporated or deemed to be incorporated by reference in this prospectus supplement and in the short form base shelf prospectus dated June 5, 2018 to which it relates, constitutes a public offering of these securities only in those jurisdictions where they may be lawfully offered for sale and therein only by persons permitted to sell such securities. See “Plan of Distribution.”
Information has been incorporated by reference in this prospectus supplement, and in the short form base shelf prospectus dated June 5, 2018 to which it relates from documents filed with securities commissions or similar authorities in Canada. Copies of the documents incorporated herein by reference may be obtained on request without charge from the Corporate Secretary of IMV Inc. at 130 Eileen Stubbs Avenue, Suite 19, Dartmouth, Nova Scotia, Canada, B3B 2C4 (telephone (902) 492-1819), and are also available electronically at www.sedar.com.
PROSPECTUS SUPPLEMENT
TO THE SHORT FORM BASE SHELF PROSPECTUS DATED JUNE 5, 2018
| New Issue | March 18, 2020 |

IMV INC.
US$30,000,000
Common Shares
IMV Inc. (“IMV” or the “Corporation”) has entered into an Equity Distribution Agreement dated March 18, 2020 (the “Equity Distribution Agreement”) with Piper Sandler & Co. (“Piper Sandler”) relating to the Corporation’s common shares (the “Common Shares”). In accordance with the terms of the Equity Distribution Agreement, the Corporation may offer and sell Common Shares having an aggregate offering price of up to US$30,000,000 (the “Offered Shares”) through Piper Sandler, as agent, under this prospectus supplement (the “Prospectus Supplement”). See “Plan of Distribution” beginning on page S-13 of this Prospectus Supplement for more information regarding these arrangements.
The Common Shares are listed on the Toronto Stock Exchange (the “TSX”) under the symbol “IMV” and on the Nasdaq Capital Market (“Nasdaq”) under the symbol “IMV”. On March 17, 2020, the last trading day of the Common Shares on the TSX and Nasdaq before the date hereof, the closing price of the Common Shares was C$2.90 and US$2.00, respectively. The TSX has conditionally approved the listing of the Offered Shares offered by this Prospectus Supplement, subject to the Corporation fulfilling all of the listing requirements of the TSX. The Nasdaq has been notified of the Offering. See “Plan of Distribution”.
Upon delivery of a placement notice by the Corporation, if any, Piper Sandler may sell the Offered Shares in the United States only and such sales will only be made by transactions that are deemed to be an “at-the-market” offering as defined in Rule 415 under the United States Securities Act of 1933, as amended (the “Securities Act”), including, without limitation, sales made directly on Nasdaq, or on any other existing trading market for the Common Shares in the United States (the “Offering”). No Offered Shares will be offered or sold in Canada or on the TSX or any other trading markets in Canada. See “Plan of Distribution” in this Prospectus Supplement. Piper Sandler will make all sales using commercially reasonable efforts consistent with their normal sales and trading practices and on mutually agreed upon terms between Piper Sandler and the Corporation. The Offered Shares will be distributed at the market prices prevailing at the time of the sale of such Offered Shares. As a result, prices may vary as between purchasers and during the period of distribution. There is no arrangement for funds to be received in escrow, trust or similar arrangement.
The compensation to Piper Sandler for sales of the Offered Shares under this Prospectus Supplement will not exceed three percent (3%) of the gross proceeds from the sale of such Offered Shares. See “Plan of Distribution” in this Prospectus Supplement. The net proceeds, if any, from sales under this Prospectus Supplement will be used as described under the section titled “Use of Proceeds” in this Prospectus Supplement. The proceeds the Corporation receives from sales will depend on the number of Offered Shares actually sold and the offering price of such Offered Shares. In connection with the sale of the Offered Shares on the Corporation’s behalf, Piper Sandler will be deemed to be an “underwriter” within the meaning of Section 2(a)(11) of the Securities Act, and the compensation of Piper Sandler will be deemed to be an underwriting commission or discount. IMV has agreed to provide indemnification and contribution to Piper Sandler against certain liabilities, including liabilities under the Securities Act.
Neither Piper Sandler, nor any of its affiliates or any person or company acting jointly or in concert with Piper Sandler, has over-allotted, or will over-allot, Common Shares in connection with this Offering or effect any other transactions that are intended to stabilize or maintain the market price of the Common Shares.
An investment in the Offered Shares involves a high degree of risk. Prospective investors should carefully consider the risk factors described in and/or incorporated by reference in this Prospectus Supplement and the Base Shelf Prospectus. See “Cautionary Statement Regarding Forward-Looking Statements” and “Risk Factors”.
The offering of Securities hereunder is made by a Canadian issuer that is permitted, under a multijurisdictional disclosure system (“MJDS”) adopted by the United States and Canada, to prepare this Prospectus Supplement and the Base Shelf Prospectus in accordance with Canadian disclosure requirements. Prospective investors should be aware that such requirements are different from those of the United States. Annual financial statements for the year ended December 31, 2018 included or incorporated herein have been prepared in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board (“IFRS”) and are subject to Canadian auditing and auditor independence standards and thus may not be comparable to financial statements of United States companies.
The enforcement by investors of civil liabilities under the United States federal securities laws may be affected adversely by the fact that the Corporation is incorporated or organized under the laws of a foreign country, that some or all of its officers and directors may be residents of a foreign country, that some or all of the experts named in this Prospectus Supplement and the Base Shelf Prospectus may by residents of a foreign country and that all or a substantial portion of the assets of the Corporation and said persons may be located outside the United States. See “Enforceability of Judgments”.
THESE SECURITIES HAVE NOT BEEN APPROVED OR DISAPPROVED BY THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION (THE “SEC”), THE SECURITIES COMMISSION OF ANY STATE OF THE UNITED STATES OR ANY CANADIAN SECURITIES COMMISION OR REGULATORY AUTHORITY NOR HAVE ANY OF THE FOREGOING PASSED UPON THE ACCURACY OR ADEQUACY OF THIS PROSPECTUS SUPPLEMENT AND THE BASE SHELF PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
Prospective investors should be aware that the acquisition, holding or disposition of the Offered Shares described herein may have tax consequences both in the United States and in Canada. Such consequences for investors who are resident in, or citizens of, the United States and Canada may not be described fully herein. You should read the tax discussion contained in this Prospectus Supplement and consult your own tax advisor with respect to your own particular circumstances. See the sections titled “Certain Canadian Federal Income Tax Considerations”, “Certain U.S. Federal Income Tax Considerations” and “Risk Factors.”
The Offered Shares may only be sold in those jurisdictions where offers and sales are permitted. This Prospectus Supplement is not an offer to sell or a solicitation of an offer to buy the Offered Shares in any jurisdiction in which it is unlawful.
ii
Julia Gregory, Wayne Pisano and Markus Warmuth, members of the board of directors of the Corporation, all reside outside of Canada and have appointed IMV Inc., 130 Eileen Stubbs Avenue, Suite 19, Dartmouth, Nova Scotia, Canada, B3B 2C4, as agent for service of process. Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process.
The Corporation’s head office and registered office is located at 130 Eileen Stubbs Avenue, Suite 19, Dartmouth, Nova Scotia, Canada, B3B 2C4.
There is no minimum amount of funds that must be raised under this offering. This means that the Corporation could complete this offering after raising only a small proportion of the offering amount set forth above.
Piper Sandler & Co.
Prospectus Supplement dated March 18, 2020
iii
TABLE OF CONTENTS
Prospectus Supplement
TABLE OF CONTENTS
Base Shelf Prospectus
ii
This document is in two parts. The first part is this Prospectus Supplement, which describes the terms of the Offering and adds to and updates information in the accompanying Base Shelf Prospectus and the documents incorporated by reference therein. The second part is the accompanying Base Shelf Prospectus, which gives more general information, some of which may not apply to the Offering. This Prospectus Supplement is deemed to be incorporated by reference into the accompanying Base Shelf Prospectus solely for the purposes of this Offering. This Prospectus Supplement may add, update or change information contained in the accompanying Base Shelf Prospectus. Before investing, you should carefully read both this Prospectus Supplement and the accompanying Base Shelf Prospectus together with the additional information about the Corporation to which you are referred in the sections of this Prospectus Supplement and the Base Shelf Prospectus titled “Documents Incorporated by Reference”.
Purchasers of Offered Shares should rely only on the information contained in or incorporated by reference into this Prospectus Supplement and the Base Shelf Prospectus. The Corporation has not authorized anyone to provide purchasers with different or additional information. If information in this Prospectus Supplement is inconsistent with the Base Shelf Prospectus or the information incorporated by reference, you should rely on this Prospectus Supplement. If anyone provides purchasers with different or additional information, purchasers should not rely on it. Neither the Corporation nor Piper Sandler are making an offer to sell or seeking an offer to buy the Offered Shares in any jurisdiction where the offer or sale is not permitted. Purchasers should assume that the information contained in this Prospectus Supplement and the Base Shelf Prospectus is accurate only as of the date on the front of those documents and that information contained in any document incorporated by reference is accurate only as of the date of that document, regardless of the time of delivery of this Prospectus Supplement and the Base Shelf Prospectus or of any sale of the Offered Shares. The Corporation’s business, financial condition, results of operations and prospects may have changed since those dates.
This Prospectus Supplement and the Base Shelf Prospectus include references to trade names and trademarks of other companies, which trade names and trademarks are the properties of their respective owners.
The corporate website of the Corporation is www.imv-inc.com. The information on the Corporation’s website is not intended to be included or incorporated by reference into this Prospectus Supplement and the Base Shelf Prospectus and prospective purchasers should not rely on such information when deciding whether or not to invest in the Offered Shares.
Statistical information and other data relating to the pharmaceutical and biotechnology industry included in this Prospectus Supplement and the Base Shelf Prospectus are derived from recognized industry reports published by industry analysts, industry associations and/or independent consulting and data compilation organizations. Market data and industry forecasts used throughout this Prospectus Supplement and the Base Shelf Prospectus were obtained from various publicly available sources. Although the Corporation believes that these independent sources are generally reliable, the accuracy and completeness of the information from such sources are not guaranteed and have not been independently verified.
This Prospectus Supplement and the Base Shelf Prospectus are part of the Corporation’s registration statement on Form F-10 (File No. 333-225326) filed with and declared effective by the U.S. Securities and Exchange Commission under the Securities Act (as amended, the “U.S. Registration Statement”). This Prospectus Supplement and the Base Shelf Prospectus do not contain all of the information set forth in the U.S. Registration Statement, certain parts of which are omitted in accordance with the rules and regulations of the SEC, or the schedules or exhibits that are part of the U.S. Registration Statement. Investors in the United States should refer to the U.S. Registration Statement and the exhibits thereto for further information with respect to IMV and the Offered Shares.
In this Prospectus Supplement, the Base Shelf Prospectus and the documents incorporated by reference herein and therein, unless the context otherwise requires, references to “IMV” or the “Corporation” refer to IMV Inc., together with its subsidiary, Immunovaccine Technologies Inc. (“IVT”).
S-1
The consolidated financial statements incorporated by reference into this Prospectus Supplement and the Base Shelf Prospectus and the other documents incorporated by reference into this Prospectus Supplement and the Base Shelf Prospectus, and the financial data derived from those consolidated financial statements included in this Prospectus Supplement and the Base Shelf Prospectus, are presented in Canadian dollars, unless otherwise specified, and have been prepared in accordance with IFRS. References in this Prospectus Supplement to “dollars”, “C$” or “$” are to Canadian dollars. United States dollars are indicated by the symbol “US$”.
The following table lists, for each period presented, the high and low exchange rates, the average of the exchange rates during the period indicated, and the exchange rates at the end of the period indicated, for one Canadian dollar, expressed in United States dollars, based on the exchange rate published by the Bank of Canada for the applicable periods. Periods prior to March 1, 2017 are based on the noon rate published by the Bank of Canada. Periods from and after March 1, 2017 are based on the closing exchange rate published by the Bank of Canada.
| Year ended December 31, | Nine Months
ended September 30, | |||||||||||||||||||
| 2019 | 2018 | 2017 | 2019 | 2018 | ||||||||||||||||
| High for the period | 0.7699 | 0.8138 | 0.8245 | 0.7670 | 0.8138 | |||||||||||||||
| Low for the period | 0.7353 | 0.7330 | 0.7276 | 0.7353 | 0.7513 | |||||||||||||||
| End of period | 0.7699 | 0.7330 | 0.7971 | 0.7551 | 0.7725 | |||||||||||||||
| Average for the period | 0.7537 | 0.7721 | 0.7708 | 0.7524 | 0.7769 | |||||||||||||||
On March 17, 2020, the closing exchange rate for one Canadian dollar, expressed in United States dollars, as reported by the Bank of Canada, was C$1.00 = US$0.7055.
Cautionary Statement Regarding Forward-Looking Statements
Certain statements contained in this Prospectus Supplement, the Base Shelf Prospectus and the documents incorporated by reference in this Prospectus Supplement and the Base Shelf Prospectus may constitute “forward-looking information” within the meaning of applicable securities laws in Canada and “forward-looking statements” within the meaning of the United States Private Securities Legislation Reform Act of 1995 (collectively, “forward-looking statements”) which involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements of the Corporation, or industry results, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. When used in this Prospectus Supplement, such statements reflect current expectations regarding future events and operating performance and speak only as of the date of this Prospectus Supplement. Forward-looking statements may use such words as “will”, “may”, “could”, “intends”, “potential”, “plans”, “believes”, “expects”, “projects”, “estimates”, “anticipates”, “continue”, “predicts” or “should” and other similar terminology.
Forward-looking statements include, but are not limited to, statements relating to:
| - | the Corporation’s business strategy; |
| - | statements with respect to the sufficiency of the Corporation’s financial resources to support its activities; |
| - | potential sources of funding; |
| - | the Corporation’s ability to obtain necessary funding on favorable terms or at all; |
| - | the Corporation’s expected expenditures and accumulated deficit level; |
| - | the Corporation’s expected outcomes from its ongoing and future research and research collaborations; |
| - | the Corporation’s ability to obtain necessary regulatory approvals; |
| - | the Corporation’s expected outcomes from its pre-clinical studies and trials; |
S-2
| - | the Corporation’s exploration of opportunities to maximize shareholder value as part of the ordinary course of its business through collaborations, strategic partnerships and other transactions with third parties; |
| - | the Corporation’s plans for the research and development of certain product candidates; |
| - | the Corporation’s strategy for protecting its intellectual property; |
| - | the Corporation’s ability to identify licensable products or research suitable for licensing and commercialization; |
| - | the Corporation’s ability to obtain licences on commercially reasonable terms; |
| - | the Corporation’s plans for generating revenue; |
| - | the Corporation’s plans for future clinical trials; |
| - | the Corporation’s expected reporting with respect to its cash equivalents and working capital as of December 31, 2019; |
| - | the Corporation’s expected use of the net proceeds from this offering, if any; and |
| - | the Corporation’s hiring and retention of skilled staff. |
The forward-looking statements reflect the Corporation’s current views with respect to future events, are subject to risks and uncertainties, and are based upon a number of estimates and assumptions that, while considered reasonable by the Corporation, are inherently subject to significant business, economic, competitive, political and social uncertainties and contingencies. Many factors could cause the Corporation’s actual results, performance or achievements to be materially different from any future results, performance, or achievements that may be expressed or implied by such forward-looking statements, including, among others:
| - | obtaining additional funding on reasonable terms when necessary; |
| - | positive results of pre-clinical studies and clinical trials; |
| - | the Corporation’s ability to successfully develop existing and new products; |
| - | the Corporation’s ability to hire and retain skilled staff; |
| - | the products and technology offered by the Corporation’s competitors; |
| - | general business and economic conditions, including as a result of the pandemic outbreak of COVID-19 |
| - | the Corporation’s ability to protect its intellectual property; |
| - | the Corporation’s ability to manufacture its products and to meet demand; |
| - | the general regulatory environment in which the Corporation operates; and |
| - | obtaining necessary regulatory approvals and the timing in respect thereof. |
Should one or more of these risks or uncertainties materialize, or should the assumptions set out in the section entitled “Risk Factors” underlying those forward-looking statements prove incorrect, actual results may vary materially from those described herein. These forward-looking statements are made as of the date of this Prospectus Supplement or the date of the Base Shelf Prospectus or, in the case of documents incorporated by reference in this Prospectus Supplement or the Base Shelf Prospectus, as of the date of such documents, and the Corporation does not intend, and does not assume any obligation, to update these forward-looking statements, except as required by law. There is no assurance that such statements will prove to be accurate as actual results and future events could differ materially from those anticipated in such statements. Purchasers are cautioned that forward-looking statements are not guarantees of future performance and accordingly purchasers are cautioned not to put undue reliance on forward-looking statements due to the inherent uncertainty therein. New factors emerge from time to time, and it is not possible for management of the Corporation to predict all of these factors or to assess in advance the impact of each such factor on the Corporation’s business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statement.
S-3
In addition, statements that “the Corporation believes” and similar statements reflect management’s beliefs and opinions on the relevant subject. These statements are based upon information available to management as of the date of this Prospectus Supplement, and while management believes such information forms a reasonable basis for such statements, such information may be limited or incomplete, and such statements should not be read to indicate that management has conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and investors are cautioned not to unduly rely upon these statements.
The forward-looking statements contained in this Prospectus Supplement and the Base Shelf Prospectus are expressly qualified by the foregoing cautionary statements and are made as of the date of this Prospectus Supplement or the Base Shelf Prospectus. The Corporation does not undertake any obligation to publicly update or revise any forward-looking statements, except as required by applicable securities laws. Purchasers should read this entire Prospectus Supplement and the Base Shelf Prospectus and consult their own professional advisors to assess the income tax, legal, risk factors and other aspects of their investment in the Offered Shares.
DOCUMENTS INCORPORATED BY REFERENCE
This Prospectus Supplement is deemed to be incorporated by reference into the Base Shelf Prospectus solely for the purpose of the Offering. Other information has also been incorporated by reference in the Base Shelf Prospectus from documents filed with the securities commissions or similar authorities in Canada. Copies of the documents incorporated herein by reference may be obtained on request without charge from the Corporate Secretary of the Corporation at 130 Eileen Stubbs Avenue, Suite 19, Dartmouth, Nova Scotia, Canada, B3B 2C4 (telephone (902) 492-1819), and are also available electronically on the Corporation’s issuer profile at www.sedar.com.
In addition to the continuous disclosure obligations of the Corporation under the securities laws of certain provinces of Canada, the Corporation is subject to certain of the information requirements of the U.S. Securities Exchange Act of 1934, as amended (the “Exchange Act”), and in accordance therewith file reports and other information with the SEC. Under MJDS, some reports and other information may be prepared in accordance with the disclosure requirements of Canada, which requirements are different from those of the United States. As a foreign private issuer, the Corporation is exempt from the rules under the Exchange Act prescribing the furnishing and content of proxy statements, and the Corporation’s officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, the Corporation may not be required to publish financial statements as promptly as U.S. companies. A free copy of any public document filed by IMV with the SEC’s Electronic Data Gathering and Retrieval (“EDGAR”) system is available from the SEC’s website at www.sec.gov.
As of the date hereof, the following documents filed with the securities commissions or similar authorities in the provinces of British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Québec, Nova Scotia and Newfoundland and Labrador are specifically incorporated by reference in and form an integral part of this Prospectus Supplement:
| (i) | the annual information form of the Corporation dated April 1, 2019 for the year ended December 31, 2018 (the “AIF”); |
| (ii) | the audited annual consolidated financial statements of the Corporation and the notes thereto for the years ended December 31, 2018 and 2017, together with the auditor’s report thereon; |
| (iv) | the unaudited interim condensed consolidated financial statements of the Corporation and the notes thereto for the nine months ended September 30, 2019 and 2018; |
S-4
| (vi) | the management information circular dated April 4, 2019 relating to the annual and special meeting of shareholders of the Corporation held on May 9, 2019; |
| (vii) | the material change report dated March 1, 2019 relating to the pricing of an underwritten public offering of Common Shares (the “2019 Public Offering”); and |
| (viii) | the material change report dated March 6, 2019 relating to the closing of the 2019 Public Offering. |
All documents of the Corporation of the type described in Section 11.1(1) of Form 44-101F1 — Short Form Prospectus to National Instrument 44-101 — Short Form Prospectus Distributions (“NI 44-101”), including any documents of the Corporation of the type referred to in the preceding paragraph and any material change reports (excluding any confidential material change reports) filed by the Corporation with a securities commission or similar regulatory authority in Canada on or after the date of this Prospectus Supplement and prior to the termination of the Offering shall be deemed to be incorporated by reference into this Prospectus Supplement and the Base Shelf Prospectus.
In addition, to the extent that any document or information incorporated by reference into this Prospectus Supplement and the Base Shelf Prospectus is included in any report on Form 6-K, Form 40-F or Form 20-F (or any respective successor form) that is filed with or furnished to the SEC by the Corporation after the date of this Prospectus, such document or information shall be deemed to be incorporated by reference as an exhibit to the U.S. Registration Statement of which this Prospectus forms a part. In addition, the Corporation may incorporate by reference into this Prospectus, or the U.S. Registration Statement of which it forms a part, other information from documents that the Corporation will file with or furnish to the SEC pursuant to Section 13(a) or 15(d) of the Exchange Act, if and to the extent expressly provided therein.
Any statement contained in this Prospectus Supplement, the Base Shelf Prospectus or in a document incorporated or deemed to be incorporated by reference in this Prospectus Supplement or the Base Shelf Prospectus shall be deemed to be modified or superseded for purposes of this Prospectus Supplement to the extent that a statement contained herein or in any other subsequently filed document which also is or is deemed to be incorporated by reference in this Prospectus Supplement or the Base Shelf Prospectus modifies or supersedes such statement. The modifying or superseding statement need not state that it has modified or superseded a prior statement or include any other information set forth in the document that it modifies or supersedes. Any statement so modified or superseded shall not be deemed to constitute a part of this Prospectus Supplement or the Base Shelf Prospectus, except as so modified or superseded.
You should rely only on the information contained in or incorporated by reference in this Prospectus Supplement and the Base Shelf Prospectus and on the other information included in the U.S. Registration Statement of which the Base Shelf Prospectus forms a part. The Corporation is not making an offer of Offered Shares in any jurisdiction where the offer is not permitted by law.
DOCUMENTS FILED AS PART OF THE U.S. REGISTRATION STATEMENT
The following documents have been or will be (through post-effective amendment or incorporation by reference) filed with the SEC as part of the U.S. Registration Statement of which this Prospectus is a part insofar as required by the SEC’s Form F-10:
| · | the documents listed under “Documents Incorporated by Reference” in this Prospectus; |
| · | the form of Equity Distribution Agreement described in this Prospectus Supplement; |
| · | the consent of PricewaterhouseCoopers LLP, the Corporation’s independent auditor; |
S-5
| · | the consent of McCarthy Tétrault LLP, the Corporation’s Canadian counsel; and |
| · | powers of attorney of the Corporation’s directors and officers, as applicable. |
| Common Shares Offered by the Corporation | Common Shares having an aggregate offering price of up to US$30,000,000 | |
| Manner of Offering | “At-the-market” offering that may be made from time to time through Piper Sandler, as sales agent. See the section titled “Plan of Distribution” on page S-13 of this Prospectus Supplement. | |
| Use of Proceeds | The Corporation intends to use the net proceeds of the Offering for general corporate purposes including but not limited to working capital expenditures, capital expenditures, research and development expenditures, including development of its vaccine candidate DPX-COVID-19, and clinical trial expenditures. See the section titled “Use of Proceeds” on page S-12 of this Prospectus Supplement. | |
| Risk Factors | Investing in the Common Shares involves a high degree of risk. Please read the information contained in and incorporated by reference under the section titled “Risk Factors” beginning on page S-16 of this Prospectus Supplement, and under similar headings in the other documents that are filed after the date hereof and incorporated by reference into this Prospectus Supplement. | |
| Nasdaq Symbol | “IMV” | |
| TSX Symbol | “IMV” |
Piper Sandler may sell the Offered Shares in the United States only and such sales will only be made by transactions that are deemed to be an “at-the-market” offering as defined in Rule 415 under the Securities Act, including, without limitation, sales made directly on Nasdaq, or on any other existing trading market for the Common Shares in the United States. No Common Shares will be offered or sold in Canada or on the TSX or any other trading markets in Canada.
The following description of IMV is derived from selected information about the Corporation contained in the documents incorporated by reference and does not contain all of the information about the Corporation and its business that should be considered before investing in the Offered Shares. This Prospectus Supplement, the accompanying Base Shelf Prospectus and the documents incorporated by reference herein and therein should be reviewed and considered by prospective purchasers in connection with their investment in the Offered Shares. This Prospectus Supplement may add to, update or change information in the accompanying Base Shelf Prospectus. You should carefully read this entire Prospectus Supplement and the accompanying Base Shelf Prospectus, including the risks and uncertainties discussed in the section titled “Risk Factors,” and the information incorporated by reference in this Prospectus Supplement, including the consolidated financial statements of the Corporation, before making an investment decision. If you invest in the Corporation’s securities, you are assuming a high degree of risk.
The Corporation was incorporated on May 18, 2007 under the name of Rhino Resources Inc. pursuant to the Canada Business Corporations Act. On September 28, 2009, the Corporation changed its name to Immunovaccine Inc. and consolidated its outstanding share capital on a 5 to 1 basis. On May 2, 2018, the Corporation changed its name to IMV Inc. and consolidated its outstanding share capital on a 3.2 to 1 basis.
The Corporation has one wholly-owned subsidiary, IVT, which is incorporated under the laws of Nova Scotia.
The Corporation’s head and registered office is located at 130 Eileen Stubbs Avenue, Suite 19, Dartmouth, Nova Scotia, Canada, B3B 2C4.
S-6
Overview
IMV is a clinical-stage biopharmaceutical company dedicated to making immunotherapy more effective, more broadly applicable, and more widely available to people facing cancer and other serious diseases. IMV is headquartered in Dartmouth, Nova Scotia and currently has 67 employees. IMV is pioneering a new class of immunotherapies based on the Corporation’s proprietary drug delivery platform (“DPX”). This patented technology leverages a novel mechanism of action (“MOA”) discovered by the Corporation. This MOA does not release the active ingredients at the site of injection but forces an active uptake by immune cells (antigen-presenting cells) and delivery of active ingredients into lymph nodes. This unique MOA enables the programming of immune cells in vivo, which are aimed at generating powerful target-specific therapeutic capabilities. DPX’s no-release MOA can be leveraged to generate “first-in-class” T cell therapies with the potential, in the opinion of IMV, to be disruptive in the treatment of cancer.
The DPX platform is based on active ingredients formulated in lipid nanoparticles and, after freeze drying, suspended directly into a lipidic formulation. DPX-based products are stored in a dry format, which provides the added benefit of an extended shelf life. The formulation is designed to be easy to re-suspend and administer to patients.
DPX also has multiple manufacturing advantages; it is fully synthetic, can accommodate hydrophilic and hydrophobic compounds, is amenable to a wide-range of applications (for example, peptides, small molecules, RNA/DNA or antibodies), and provides long-term stability.
The DPX platform forms the basis of all IMV’s product development programs.
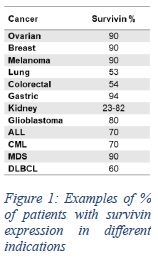
The Corporation’s first cancer immunotherapy uses survivin-based peptides licensed from Merck KGaA, on a world-wide exclusive basis, formulated in DPX (“DPX-Survivac”). DPX-Survivac leverages the MOA of DPX to generate a constant flow of T cells in the blood that are targeted against survivin expressed on cancer cells. It is comprised of five minimal MHC class I peptides to activate naïve T cells against survivin.
The Corporation’s first cancer immunotherapy uses survivin-based peptides licensed from Merck KGaA, on a world-wide exclusive basis, formulated in DPX (“DPX-Survivac”). Survivin is a well characterized and tumor-associated antigen known to be overexpressed in more than 20 different cancers. DPX-Survivac leverages the MOA of the DPX platform to generate a constant flow of killer T cells in the blood that are targeted against survivin expressed on cancer cells. It is comprised of five minimal MHC class I peptides to activate naïve T cells against survivin.
Foremost, the Corporation’s clinical strategy is to target late stage unmet medical needs for a potentially shorter path to clinical demonstration and first regulatory approval. In addition, the Corporation is evaluating potential combinations with checkpoint inhibitor Keytruda® (pembrolizumab) of Merck & Co Inc. (“Merck”) in multiple solid tumor indications, as discussed below. |
 |
The Corporation is focusing on a path to market in ovarian and diffuse large B cell lymphoma (“DLBCL”) cancers and on repeating its clinical demonstrations of activity in other indications.
The Corporation has completed Phase 1 and 1b clinical trials in ovarian cancer in a maintenance setting after first or second line in 56 subjects.
The results provided first in human clinical demonstration of DPX-Survivac mechanism of action (survivin-specific T cell flow in the blood) and potential as treatment. 87% of patients generated one of the highest T cell levels reported in the literature and that level was maintained during a year with repeated injection every two months.
S-7
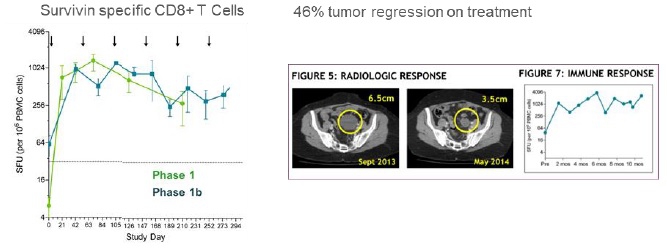
Figure 2: Phase 1/1b result (ASCO 2015)
DPX-Survivac is currently being tested in:
| · | a phase 2 open label clinical trial which evaluates DPX-Survivac in patients with advanced platinum-sensitive and resistant ovarian cancer with sum of baseline target lesions per RECIST criteria less than five centimeters; |
| · | two investigator-sponsored phase 2 clinical trials in combination with Merck’s checkpoint inhibitor Keytruda® (pembrolizumab) in patients with recurrent, platinum-resistant and sensitive ovarian cancer and in patients with measurable or recurrent DLBCL. In these cases, the primary and secondary endpoints include objective response rate (“ORR”), tumor regression, toxicity profile and duration of responses; and |
| · | a phase 2 basket trial in combination with Merck’s Keytruda® (pembrolizumab), in five different indications in patients with select advanced or recurrent solid tumours in bladder, liver (“hepatocellular carcinoma”), ovarian or non-small-cell lung (“NSCLC”) cancers, as well as tumours shown to be positive for the microsatellite instability high (“MSI-H”) biomarker. |
S-8
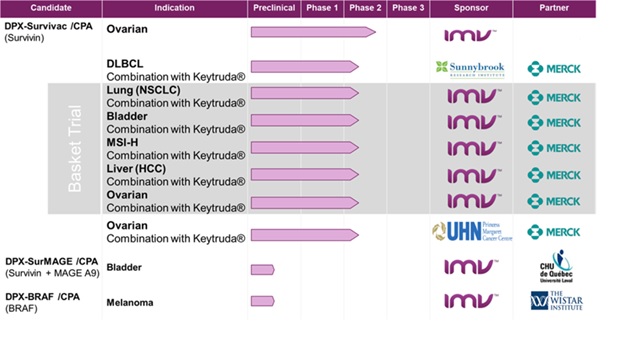
Figure 3: Ongoing phase 2 clinical trials
In infectious disease vaccine applications, the Corporation has completed a demonstration phase 1 clinical trial with a target against the respiratory syncytial virus (“RSV”). The Corporation also has a commercial licensing agreement with Zoetis for the development of two targeted therapies for cattle and is also conducting several research and clinical collaborations, including a collaboration with the Dana-Farber Cancer Institute for Human Papillomavirus (“HPV”) related cancers and with Leidos, Inc. in the United States for the development of targeted therapies for malaria and the Zika virus.
For further information, see “Description of the Business” in the AIF.
Phase 2 SPiReL Study
On December 8, 2019, the Corporation announced that updated results from SPiReL, an ongoing Phase 2 investigator-sponsored study of DPX-Survivac in combination with pembrolizumab in patients with recurrent/refractory diffuse large B-cell lymphoma (“r/r DLBCL”), were presented in a poster session at the 61st American Society of Hematology (“ASH”) Annual Meeting in Orlando, FL. The poster, which included additional data collected between the abstract submission and the presentation, continued to demonstrate a favorable therapeutic profile and treatment-associated clinical benefit in r/r DLBCL patients who received the combination therapy with DPX-Survivac.
In the poster presentation at ASH, Dr. Neil Berinstein, MD, FFCPC, ABIM, hematologist at Sunnybrook Health Sciences Centre and lead investigator for the clinical trial, reported updated clinical results from the ongoing Phase 2 SPiReL study. Highlights of this preliminary data are outlined below:
| · | 7/9 (77.8%) evaluable subjects exhibited clinical benefit, including three (33.3%) complete responses and two (22.2%) partial responses; |
| · | Reproducible survivin-specific T cell responses observed in all subjects that achieved clinical responses on treatment; |
S-9
| · | One subject, who received three prior lines of systemic therapies and failed autologous stem cell transplant, reached a complete response at the first on-study scan following treatment with the DPX-Survivac combination regimen and remains free of disease recurrence after completing the study; and |
| · | Clinical benefits and favorable toxicity profile observed in a heterogenous population of r/r DLBCL patients, including patients of advanced age and/or with comorbidities, who are more susceptible to adverse effects and more difficult to treat. |
As of December 1, 2019, 17 subjects had been enrolled in the study.
Clinical Translational Data
On February 4, 2020, IMV announced that clinical translational data supporting the MOA of DPX-Survivac, would be presented during the 2020 ASCO-SITC Clinical Immuno-Oncology Symposium being held in early February 2020 in Orlando, FL. As part of this analysis, the Corporation measured systemic immune responses, tumor immune infiltrates and clinical tumor response from pre- and post-treatment patient samples in connection with three Phase 1 and/or Phase 2 clinical studies, each evaluating DPX-Survivac alone or in a combination with pembrolizumab in patients with platinum sensitive or resistant, advanced ovarian cancer. Highlights from these translational data include:
| · | DPX-Survivac generated survivin-specific T cells in the blood of 80% of patients sampled; |
| · | Clinical anti-tumor responses were correlated with increased infiltration of T cells into tumors following treatment with DPX-Survivac; |
| · | DPX-Survivac induced enrichment in T cell, cytotoxic lymphocytes and B cell-specific signatures which correlate with clinical response; and |
| · | Antigen-specific T cells retained their functionality throughout the duration of treatment. |
Board retirement
On February 14, 2020, IMV announced that Albert Scardino would retire from the board of directors of the Corporation effective February 28, 2020.
Phase 2 DeCidE1 Study
On February 25, 2020, IMV reported updated results from DeCidE1, an ongoing Phase 2 study of its lead candidate, DPX-Survivac, in patients with advanced recurrent ovarian cancer. The new results show that DPX-Survivac immunotherapy is active and well-tolerated in patients with advanced ovarian cancer.
As of February 24, 2020, 19 patients were evaluable for efficacy with six patients (31%) still receiving treatment. Key preliminary findings are outlined below:
| · | 15 patients (79%) achieved disease control, defined as Stable Disease (“SD”) or Partial Response (“PR”) on target lesions: |
| o | Tumor shrinkage of target lesions was observed in 10 patients (53%). |
| · | Durable clinical benefits lasting ≥6 months were observed in seven patients (37%) so far: |
| o | Four of these seven patients (21% of evaluable patients) achieved PR with tumor regression >30% on target lesions; |
| o | Three stable diseases were ongoing for > 6 months (range 7-9) including -29.5% and -12% tumor regressions; and |
| o | Median duration not reached yet, with five of these seven (71%) patients still on treatment at > 6 months (range 7-10). |
S-10
| · | Analysis of Baseline Tumor Burden (“BTB”) showed durable clinical benefits across a broad range of BTB (1.5-7.7 cm) with a higher number of patients achieving benefits in BTB < 5 cm as previously observed in other arms of the study: |
| o | Six out 11 with BTB < 5 cm (55%) achieved clinical benefits lasting > 6 months. |
| · | Durable clinical benefits include platinum-resistant and refractory patients who previously received PARP inhibitors and bevacizumab. |
| · | Treatment was well-tolerated, with most adverse events being Grade 1-2 reactions at the injection site. |
The Corporation plans to take these results to the U.S. Food and Drug Administration (“FDA”) for a Type B meeting, to align on the design of a Phase 2b study with potential to support registration under accelerated approval in this indication.
DPX-COVID-19
The ongoing pandemic outbreak of COVID-19 and its alarmingly quick transmission to over 125 countries across the world resulted in the World Health Organization (WHO) declaring a pandemic on March 11, 2020.
The outbreak is caused by a novel coronavirus, the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). There is an urgent need to develop vaccines to control its spread and help protect vulnerable populations. However, the bottleneck with current conventional vaccine approaches is the length of time required for vaccine development. We believe IMV’s DPX delivery technology offers the possibility of a fully synthetic epitope-based approach with the potential for accelerated development and rapid, large-scale production of a vaccine that would be compliant with current good manufacturing practice (cGMP).
Research in coronaviruses has identified the benefit of humoral and cellular (B and T cell) immune responses for treatment and protection from infection.
IMV believes that it has already demonstrated in multiple clinical trials in oncology and infectious diseases the potential of its technology for the induction of robust and sustained B and T cells. The Corporation believes there is an opportunity to pursue a COVID-19 development program to establish the clinical safety and immunogenicity using a similar approach for COVID-19.
IMV intends to develop its vaccine candidate DPX-COVID-19 in collaboration with lead investigators for the phase 1 clinical study: Joanne Langley, M.D. and Scott Halperin, M.D., of the Canadian Center for Vaccinology (CCfV) at Dalhousie University, the Izaak Walton Killam Health Center and the Nova Scotia Health Authority and the Canadian Immunization Research Network (CIRN); along with Dr. Gary Kobinger, Ph.D., Director of the Research Centre on Infectious Diseases at the University Laval in Quebec City and Global Urgent and Advanced Research and Development (GUARD) in Canada. The investigators will assist with preclinical and clinical evaluation and with further development strategy in collaboration with the Canadian government and others.
Third-party research in related coronaviruses has identified the benefit of humoral and cellular (B and T cell) immune responses for protection and resolution of infection, and the Company believes the body of data it has produced to date supports its DPX platform for peptide-based induction of B cells and T cells. The Corporation is now designing a vaccine candidate against COVID-19 based on third-party immunological studies of SARS-CoV and third-party sequencing data available for SARS-CoV-2 with the goal of selecting potentially immunogenic epitopes within the virus that induce neutralizing antibody responses and protective T cell responses.
Through the Corporation’s other clinical studies, the Corporation believes its DPX technology has demonstrated a favorable safety profile and immunogenicity in both cancer and infectious disease settings, with sustained effect and potential for single-dose effectiveness as a prophylactic vaccine. Over 200 patients have been dosed with DPX-based immunotherapies and data from these studies suggest treatment is well-tolerated, including in heavily pre-treated cancer patients with advanced-stage disease. The Corporation has also applied this technology for the prevention of respiratory syncytial virus (RSV), the second-leading cause of respiratory illness in infants, the elderly and the immunosuppressed. The Corporation reported its Phase 1 data from its clinical candidate, DPX-RSV, which demonstrated a favorable safety profile and immunogenicity in older adults (age 50-64), as well as preclinical data from research-stage candidates aimed at other infectious diseases, including malaria and anthrax.
S-11
Expected Fiscal Year 2019 Earnings Results
While the Corporation has not yet finalized its full financial results for the fiscal year ended December 31, 2019, IMV expects to report that it had as of December 31, 2019, cash and cash equivalents of $14.9 million and working capital of $12.2 million. This amount is preliminary, has not been audited and is subject to change upon completion of the audited financial statements for the year ended December 31, 2019.
Since September 30, 2019, the end of the most recent interim reporting period of the Corporation, there have been no material changes in the long term debt of the Corporation and no material changes in the share capital of the Corporation on a consolidated basis other than as outlined under “Prior Sales”. For information on the exercise of options pursuant to the stock option plan of the Corporation and other outstanding convertible securities, see the section titled “Prior Sales”.
The net proceeds from the Offering, if any, are not determinable in light of the nature of the distribution. The net proceeds of any given distribution of Offered Shares through Piper Sandler in an “at-the-market offering” will represent the gross proceeds after deducting the compensation payable to Piper Sandler under the Equity Distribution Agreement and expenses of the distribution. Piper Sandler will receive a cash fee not exceeding three percent (3%) of the gross proceeds realized from the sale of the Offered Shares for services rendered in connection with the Offering. The Corporation estimates the total expenses of this Offering, excluding the fee paid to Piper Sandler, will be approximately US$200,000. All expenses relating to the Offering will be paid out of the proceeds from the sale of Offered Shares, unless otherwise stated in this Prospectus Supplement.
The Corporation has negative operating cash flow and it is expected that the proceeds from the Offering will be used to fund operating cash flow. The Corporation may sell Offered Shares for aggregate gross proceeds of up to US$30,000,000. Because there is no minimum amount of our Common Shares that must be sold pursuant to the Offering, the actual number of Offered Shares sold and aggregate proceeds to us, if any, are not presently determinable and may be substantially less than the amounts set forth above.
The Corporation intends to use the net proceeds of the Offering, if any, for general corporate purposes including but not limited to working capital expenditures, capital expenditures, research and development expenditures, including development of its vaccine candidate DPX-COVID-19, and clinical trial expenditures.
While the Corporation intends to spend the net proceeds of the Offering, if any, as stated above, there may be circumstances where, for sound business reasons, a re-allocation of funds may be necessary or advisable. The actual amount that the Corporation spends in connection with the intended uses of proceeds may vary significantly , and will depend on a number of factors, including those listed under the heading “Risk Factors” in this Prospectus Supplement and the accompanying Base Shelf Prospectus and the documents incorporated by reference herein and therein.
Negative Cash Flow
The Corporation has incurred significant operating losses and negative cash flows from operations since inception and has an accumulated deficit of C$111,644,000 as of September 30, 2019. The ability of the Corporation to continue as a going concern is dependent upon raising additional financing through equity and non-dilutive funding and partnerships. There can be no assurance that the Corporation will have sufficient capital to fund its ongoing operations, develop or commercialize any products without future financings. These material uncertainties cast significant doubt as to the Corporation’s ability to meet its obligations as they come due and, accordingly, the appropriateness of the use of accounting principles applicable to a going concern. If the Corporation is unable to obtain additional financing when required, the Corporation may have to substantially reduce or eliminate planned expenditures or the Corporation may be unable to continue operations.
S-12
The Corporation’s ability to continue as a going concern is dependent upon its ability to fund its research and development programs and defend its patent rights. It is expected that proceeds from the Offering will be used to fund anticipated negative cash flow from operating activities, as described above.
The Corporation has entered into the Equity Distribution Agreement with Piper Sandler under which it may issue and sell from time to time up to US$30,000,000 of Offered Shares through Piper Sandler, as agent. Sales of the Offered Shares, if any, will be made in transactions that are deemed to be an “at-the-market offering” as defined in Rule 415 under the Securities Act, including sales made directly on the Nasdaq, or on any other existing trading market for the Common Shares in the United States. Piper Sandler will use commercially reasonable efforts to sell the Offered Shares directly on the Nasdaq or other existing trading markets in the United States requested to be sold by the Corporation, consistent with Piper Sandler’s normal trading and sales practices, under the terms and subject to the conditions set forth in the Equity Distribution Agreement. No Offered Shares will be offered and sold in Canada. The Corporation and Piper Sandler may suspend the offering of Offered Shares upon proper notice and subject to other conditions, as specified in the Equity Distribution Agreement.
No Offered Shares will be sold in Canada or on the TSX or any other trading markets in Canada. Neither the Corporation nor Piper Sandler will undertake any act, advertisement, solicitation, conduct or negotiation directly or indirectly in furtherance of the sale of the Offered Shares in Canada, undertake an offer or sale of any of the Offered Shares to a person that it knows or has reason to believe is in Canada or has been pre-arranged with a buyer in Canada, or to any person who it knows or has reason to believe is acting on the behalf of persons in Canada or to any person whom it knows or has reason to believe intends to reoffer, resell or deliver the Offered Shares in Canada on the TSX or on other trading markets in Canada or to any persons in Canada or acting on behalf of persons in Canada.
To compensate Piper Sandler for services in acting as agent in the sale of Offered Shares, IMV will pay a commission not to exceed three percent (3%) of the gross sales price realized from the sale of the Offered Shares. The Corporation has also agreed to reimburse Piper Sandler for certain specified expenses, including the fees and disbursements of its legal counsel, in an amount not to exceed US$75,000, including fees and disbursements of Piper Sandler’s counsel incident to any required review by the Financial Industry Regulatory Authority. IMV estimates that the total expenses for the Offering, excluding compensation and reimbursements payable to Piper Sandler under the terms of the Equity Distribution Agreement, will be approximately US$200,000.
Settlement for sales of the Offered Shares will occur on the second business day following the date on which any sales are made, or on such other date as is industry practice for regular-way trading, in return for payment of the net proceeds to the Corporation. Sales of our Common Shares as contemplated in this Prospectus Supplement will be settled through the facilities of The Depository Trust Company or by such other means as we and the Agent may agree upon. There is no arrangement for funds to be received in escrow, trust or similar arrangement.
In connection with the sale of the Offered Shares on the Corporation’s behalf, Piper Sandler will be deemed to be an “underwriter” within the meaning of the Securities Act, and the compensation of Piper Sandler will be deemed to be an underwriting commission or discount. The Corporation has agreed to provide indemnification and contribution to Piper Sandler against certain civil liabilities, including liabilities under the Securities Act.
The Offering will terminate upon the issuance and sale of all Offered Shares subject to the Equity Distribution Agreement by Piper Sandler. The Corporation and Piper Sandler may also terminate the Equity Distribution Agreement under the circumstances specified in the Equity Distribution Agreement.
As sales agent, Piper Sandler will not engage in any transactions that stabilize the price of the Common Shares. No underwriter or dealer involved in the Offering, no affiliate of such an underwriter or dealer, and no person or company acting jointly or in concert with such an underwriter or dealer has over-allotted, or will over-allot, Common Shares in connection with the Offering or effect any other transactions that are intended to stabilize or maintain the market price of the Common Shares.
S-13
The Common Shares are listed on the Nasdaq and the TSX under the symbol “IMV”. The Nasdaq has been notified of the Offering, and the TSX has conditionally approved the listing of the Offered Shares offered by this Prospectus Supplement, subject to the Corporation fulfilling all of the listing requirements of the TSX set out in the conditional approval letter.
Piper Sandler and its affiliates may in the future provide various investment banking, commercial banking and other financial services for IMV and its affiliates, for which services they may in the future receive customary fees. To the extent required under the Exchange Act, Piper Sandler will not engage in any market making activities involving the Common Shares while the Offering is ongoing under the Prospectus Supplement.
The Prospectus Supplement in electronic format may be made available on a website maintained by Piper Sandler and Piper Sandler may distribute this Prospectus Supplement and the accompanying Base Shelf Prospectus electronically.
Under no circumstances will any Offered Shares be sold pursuant to the Equity Distribution Agreement after July 5, 2020, which is 25 months after June 5, 2018 (the date that the receipt was issued for the Base Prospectus).
DESCRIPTION OF securities being distributed
IMV’s authorized share capital consists of an unlimited number of Common Shares and preferred shares (the “Preferred Shares”) issuable in series, all without par value. As of September 30, 2019, a total of 50,630,875 Common Shares and no Preferred Shares were issued and outstanding. 1,556,744 Common Shares were issuable upon the exercise of outstanding stock options, 134,766 Common Shares were issuable upon the exercise of outstanding warrants and 323,903 Common Shares were issuable upon the conversion of outstanding deferred share units (“DSUs”).
The Common Shares of the Corporation rank junior to the Preferred Shares with respect to the payment of dividends, return of capital and distribution of assets in the event of liquidation, dissolution or winding-up of the Corporation. Subject to the prior rights of the holders of Preferred Shares, the holders of Common Shares are entitled to receive dividends as and when declared by the Board of Directors of the Corporation. In the event of liquidation, dissolution or winding-up of the Corporation, subject to the prior rights of the holders of Preferred Shares, the holders of Common Shares are entitled to receive all the remaining property and assets of the Corporation. The holders of Common Shares are entitled to receive notice of and to attend and to vote at all meetings of the shareholders of the Corporation and each Common Share, when represented at any meeting of the shareholders of the Corporation, carries the right to one vote.
The Corporation has not paid any dividends to date on the Common Shares. The Corporation does not currently expect to pay any dividends on the Common Shares for the foreseeable future.
The following table sets out the details of the issuance by the Corporation of Common Shares, options to purchase Common Shares, warrants to purchase Common Shares, DSUs, if any, during the 12-month period before the date of this Prospectus Supplement:
| Security | Number | Price | Issuance Date | |||||||
| Common Shares(1) | 4,900,000 | C$ | 5.45 | March 6, 2019 | ||||||
| Common Shares(1) | 504,855 | C$ | 5.45 | March 11, 2019 | ||||||
| Common Shares(2) | 2,187 | C$ | 3.20 | March 29, 2019 | ||||||
| Common Shares(2) | 859 | N/A | (3) | March 29, 2019 | ||||||
| Common Shares(2) | 14,819 | N/A | (4) | June 3, 2019 | ||||||
| Common Shares(2) | 9,375 | C$ | 2.112 | September 11, 2019 | ||||||
| Common Shares(2) | 9,375 | C$ | 2.112 | September 18, 2019 | ||||||
| Stock options(5) | 100,000 | C$ | 3.95 | November 7, 2019 | ||||||
| Common Shares(2) | 37,386 | N/A | (6) | January 28, 2020 | ||||||
| Common Shares(2) | 15,625 | C$ | 2.112 | January 29, 2020 | ||||||
| Common Shares(2) | 2,928 | N/A | (7) | January 29, 2020 | ||||||
| Common Shares(2) | 10,112 | N/A | (8) | January 30, 2020 | ||||||
| Stock options(9) | 245,850 | C$ | 5.98 | January 30, 2020 | ||||||
| Common Shares(2) | 11,213 | N/A | (10) | January 31, 2020 | ||||||
S-14
| (1) | Common Shares issued pursuant to the 2019 Public Offering. |
| (2) | Common Shares issued upon exercise of stock options. |
| (3) | Cashless exercise of 2,344 options. |
| (4) | Cashless exercise of 24,407 options. |
| (5) | Grant of stock options governed by the Corporation’s stock option plan exercisable at a price of C$3.95 per Common Share until November 7, 2024. |
| (6) | Cashless exercise of 62,500 options. |
| (7) | Cashless exercise of 4,688 options. |
| (8) | Cashless exercise of 15,625 options. |
| (9) | Grant of stock options governed by the Corporation’s stock option plan exercisable at a price of C$5.98 per Common Share until January 30, 2025. |
| (10) | Cashless exercise of 17,188 options. |
The Common Shares are currently listed on the TSX under the symbol “IMV” and Nasdaq under the symbol “IMV”.
The following table provides the price ranges and trading volume of the Common Shares on the TSX for the periods indicated below:
| Price Ranges | ||||||||||||
| High | Low | Total Cumulative Volume | ||||||||||
| March 2019 | C$ | 6.00 | C$ | 4.78 | 1,462,493 | |||||||
| April 2019 | C$ | 5.53 | C$ | 3.95 | 1,207,785 | |||||||
| May 2019 | C$ | 6.17 | C$ | 4.86 | 1,167,399 | |||||||
| June 2019 | C$ | 5.91 | C$ | 3.58 | 1,446,270 | |||||||
| July 2019 | C$ | 4.60 | C$ | 3.55 | 592,613 | |||||||
| August 2019 | C$ | 4.20 | C$ | 3.06 | 593,529 | |||||||
| September 2019 | C$ | 4.40 | C$ | 3.22 | 397,164 | |||||||
| October 2019 | C$ | 4.10 | C$ | 3.01 | 689,477 | |||||||
| November 2019 | C$ | 3.95 | C$ | 2.77 | 1,003,585 | |||||||
| December 2019 | C$ | 4.10 | C$ | 3.30 | 748,590 | |||||||
| January 2020 | C$ | 6.52 | C$ | 3.70 | 1,255,811 | |||||||
| February 2020 | C$ | 6.69 | C$ | 2.87 | 2,630,254 | |||||||
| March 1-17, 2020 | C$ | 4.50 | C$ | 2.45 | 1,888,294 | |||||||
On March 17, 2020, the last trading day of the Common Shares on the TSX before the date of this Prospectus Supplement, the closing price of the Common Shares was C$2.90.
The following table provides the price ranges and trading volume of the Common Shares on Nasdaq for the periods indicated below:
| Price Ranges | ||||||||||||
| High | Low | Total Cumulative Volume | ||||||||||
| March 2019 | US$ | 4.37 | US$ | 3.60 | 1,482,792 | |||||||
| April 2019 | US$ | 4.06 | US$ | 2.90 | 480,834 | |||||||
| May 2019 | US$ | 4.57 | US$ | 3.56 | 669,721 | |||||||
| June 2019 | US$ | 4.50 | US$ | 2.69 | 703,778 | |||||||
| July 2019 | US$ | 3.82 | US$ | 2.72 | 305,853 | |||||||
| August 2019 | US$ | 3.13 | US$ | 2.25 | 143,136 | |||||||
| September 2019 | US$ | 3.31 | US$ | 2.48 | 121,673 | |||||||
| October 2019 | US$ | 3.16 | US$ | 2.29 | 109,836 | |||||||
| November 2019 | US$ | 3.19 | US$ | 2.11 | 205,451 | |||||||
| December 2019 | US$ | 3.11 | US$ | 2.52 | 534,473 | |||||||
| January 2020 | US$ | 4.93 | US$ | 2.85 | 1,158,669 | |||||||
| February 2020 | US$ | 5.12 | US$ | 2.13 | 2,055,492 | |||||||
| March 1-17, 2020 | US$ | 3.36 | US$ | 1.76 | 1,922,255 | |||||||
S-15
On March 17, 2020, the last trading day of the Common Shares on Nasdaq before the date of this Prospectus Supplement, the closing price of the Common Shares was US$2.00.
An investment in the Corporation’s securities involves risk. Before you invest in the Offered Shares, you should carefully consider the risks contained in or incorporated by reference into this Prospectus Supplement and the Base Shelf Prospectus, including the risks described below and in the AIF and Annual MD&A, which are incorporated by reference into this Prospectus Supplement and the Base Shelf Prospectus. The discussion of risks related to the business of the Corporation contained in or incorporated by reference into this Prospectus Supplement and the Base Shelf Prospectus comprises material risks of which the Corporation is aware. If any of the events or developments described actually occurs, the business, financial condition or results of operations of the Corporation would likely be adversely affected.
Risks Relating to the Corporation
Risks Related to the Financial Position and Need for Additional Capital
The Corporation has incurred significant losses since inception and expects to incur losses for the foreseeable future and may never achieve or maintain profitability.
Since inception, the Corporation has incurred significant operating losses. The net loss was $21.9 million for the year ended December 31, 2018, $12.0 million for the year ended December 31, 2017 and $8.9 million for the year ended December 31, 2016. As of September 30, 2019, the Corporation had an accumulated deficit of $111.6 million. To date, the Corporation has financed operations primarily through public offerings in Canada, private placements of securities, grants and license and collaboration agreements. The Corporation has devoted substantially all efforts to research and development, including clinical trials. IMV expects to continue to incur significant expenses and increasing operating losses for at least the next several years. The Corporation anticipates that the expenses will increase substantially if and as the Corporation:
| · | initiates or continues the clinical trials of DPX Survivac and other product candidates, such as DPX-SurMAGE, DPX-BRAF and DPX-COVID-19; |
| · | seeks regulatory approvals for the product candidates that successfully complete clinical trials; |
| · | establishes a sales, marketing and distribution infrastructure to commercialize products for which the Corporation may obtain regulatory approval; |
| · | maintains, expands and protects the Corporation’s intellectual property portfolio; |
| · | continues other research and development efforts; |
| · | hires additional clinical, quality control, scientific and management personnel; and |
S-16
| · | adds operational, financial and management information systems and personnel, including personnel to support product development and planned commercialization efforts. |
To become and remain profitable, the Corporation must develop and eventually commercialize a product or products with significant market potential. This development and commercialization will require the Corporation to be successful in a range of challenging activities, including successfully completing preclinical testing and clinical trials of the product candidates, obtaining regulatory approval for these product candidates and marketing and selling those products that obtain regulatory approval. The Corporation is only in the preliminary stages of some of these activities. The Corporation may never succeed in these activities and may never generate revenues that are significant or large enough to achieve profitability. Even if profitability is achieved, the Corporation may not be able to sustain or increase profitability on a quarterly or annual basis. Failure to become and remain profitable would decrease the value of the Corporation and could impair the Corporation’s ability to raise capital, expand the business, maintain research and development efforts or continue operations. A decline in the value of the Corporation could also cause shareholders to lose all or part of their investment.
The Corporation will need substantial additional funding. If the Corporation is unable to raise capital when needed, the Corporation would be forced to delay, reduce, terminate or eliminate product development programs, potentially including the ongoing and planned clinical trials of its products or its commercialization efforts.
The Corporation expects expenses to increase in connection with the ongoing activities, particularly as the Corporation continues the research, development and clinical trials of, and seeks regulatory approval for, the product candidates. In addition, if the Corporation obtains regulatory approval of any of the product candidates, the Corporation expects to incur significant commercialization expenses for product sales, marketing, manufacturing and distribution. Furthermore, the Corporation will need to obtain additional funding in connection with continuing operations. If the Corporation is unable to raise capital when needed or on attractive terms, the Corporation would be forced to delay, reduce, terminate or eliminate the product development programs, potentially including the ongoing and planned clinical trials of DPX Survivac.
As of December 31, 2019, the Corporation had cash and cash equivalents of $14.9 million and working capital of $12.2 million. This amount is preliminary, has not been audited and is subject to change upon completion of the audited financial statements for the year ended December 31, 2019.
The Corporation will need to obtain significant financing prior to the commercialization of any of its products, including funding to complete all of the required clinical trials related to such products. The Corporation does not currently have funds available to enable the Corporation to complete all of the required clinical trials for the commercialization of DPX Survivac and to fund operating expenses through the completion of these trials. The Corporation expects that it will require more than $50 million or more to conduct the clinical trials and fund operating expenses through the completion of these ongoing trials.
The Corporation’s future capital requirements will depend on many factors, including:
| · | the progress and results of the clinical trials of DPX Survivac and other product candidates; |
| · | the scope, progress, results and costs of preclinical development, laboratory testing and clinical trials for other product candidates; |
| · | the costs, timing and outcome of regulatory review of any product candidate; |
| · | the costs of commercialization activities, including product sales, marketing, manufacturing and distribution, for any of the product candidates for which regulatory approval is received; |
| · | revenue, if any, received from commercial sales of the Corporation’s product candidates, should any of the product candidates be approved by the FDA, Health Canada or a similar regulatory authority outside the United States and Canada; |
S-17
| · | the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing the Corporation’s intellectual property rights and defending intellectual property related claims; |
| · | the extent to which the Corporation acquires or invests in other businesses, products and technologies; |
| · | the Corporation’s ability to obtain government or other third party funding; and |
| · | the Corporation’s ability to establish collaborations on favorable terms, if at all, particularly arrangements to market and distribute product candidates on a worldwide basis. |
Conducting preclinical testing and clinical trials is a time consuming, expensive and uncertain process that takes years to complete, and the Corporation may never generate the necessary data or results required to obtain regulatory approval and achieve product sales. In addition, the Corporation’s product candidates, if approved, may not achieve commercial success. The Corporation’s commercial revenues, if any, will be derived from sales of products that the Corporation does not expect to be commercially available for several years, if at all. Accordingly, the Corporation will need to continue to rely on additional financing to achieve the Corporation’s business objectives. Additional financing may not be available on acceptable terms to the Corporation, or at all.
Raising additional capital may cause dilution to existing shareholders, restrict operations or require the Corporation to relinquish rights to its technologies or product candidates.
Until such time, if ever, as the Corporation can generate substantial product revenues, the Corporation expects to finance its cash needs through a combination of equity offerings, debt financings, government or other third party funding, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements. Currently, the Corporation does not have any committed external source of funds. The Corporation will require substantial funding to complete the ongoing and planned clinical trials of DPX Survivac and other product candidates and to fund operating expenses and other activities. To the extent that the Corporation raises additional capital through the sale of equity or convertible debt securities, the shareholders ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the shareholders rights as a stockholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting the Corporation’s ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If the Corporation raises additional funds through government or other third party funding, marketing and distribution arrangements or other collaborations, strategic alliances or licensing arrangements with third parties, the Corporation may have to relinquish valuable rights to its technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable.
Risks Related to the Development and Commercialization of the Corporation’s Product Candidates
The Corporation does not have governmental authorization to begin clinical testing of DPX-COVID-19, and the process of conducting necessary clinical studies, manufacturing and clinical organization, as well as obtaining such governmental authorization from Health Canada is not guaranteed.
The Corporation is at the early stages of developing its proposed vaccine candidate DPX-COVID-19. Creating a new vaccine, testing it for toxicity and efficacy, securing clinical drug supply, scaling production and manufacturing, and establishing supply and distribution logistics are all steps that have significant natural time limitations. We have not received any authorization from Health Canada or any other governmental regulatory authority, to develop or initiate clinical trials for DPX-COVID-19, and although we have identified lead clinical investigators, the Corporation has not entered into any agreements for the establishment of clinical sites. Even if Health Canada were to accelerate the approval processes necessary to permit the Corporation to commence a phase 1 study and subsequent studies and trials to the maximum extent possible, the spread of the Coronavirus pandemic may be faster than its development efforts. There is no guarantee that even if the development of DPX-COVID-19 is successful, the Corporation will secure the necessary regulatory approval for its commercialization or that DPX-COVID-19 will receive market acceptance or reach the population in time. In addition, a number of other biotechnology companies, academic institutions and governmental entities are also researching and developing therapies and vaccines to address the COVID-19 pandemic, and many of these competitors have significantly greater financial and scientific resources than the Corporation. In light of the declaration by the World Health Organization of the pandemic, the third-party clinical investigators and clinical site operators that the Corporation may seek to collaborate with on the development of DPX-COVID-19, as well as governmental entities, may decide to prioritize or rationalize their resources in favor of competing therapies and vaccines. In such event, the Corporation’s efforts to develop DPX-COVID-19 could be delayed, which could harm the viability of this development program.
S-18
The Corporation depends heavily on the success of DPX Survivac and other product candidates. All of the product candidates are still in preclinical or clinical development. Clinical trials of any product candidate may not be successful. If the Corporation is unable to commercialize any product candidate or experiences significant delays in doing so, the business may be materially harmed.
All of the product candidates of the Corporation are still in preclinical or clinical development. The Corporation may never be able to obtain regulatory approval for any of its product candidates. The Corporation has committed significant human and financial resources to the development of DPX Survivac, and the DPX Platform. The ability to generate product revenues, which is not expected to occur for at least the next several years, if ever, will depend heavily on the successful development and eventual commercialization of these product candidates, especially DPX Survivac, the most advanced product candidate. The success of these product candidates will depend on several factors, including the following:
| · | successful completion of preclinical studies and clinical trials; |
| · | receipt of marketing approvals from the FDA, Health Canada and similar regulatory authorities outside the United States and Canada; |
| · | establishing commercial manufacturing capabilities by identifying and making arrangements with third party manufacturers for the product candidates; |
| · | maintaining patent and trade secret protection and regulatory exclusivity for the product candidates; |
| · | launching commercial sales of the products, if and when approved, whether alone or in collaboration with others; |
| · | acceptance of the products, if and when approved, by patients, the medical community and third party payors; |
| · | effectively competing with other therapies; and |
| · | a continued acceptable safety profile of the products following approval. |
If the Corporation does not achieve one or more of these factors in a timely manner or at all, the Corporation could experience significant delays or an inability to successfully commercialize its product candidates, which would materially harm its business.
If clinical trials of the product candidates, such as the ongoing and planned clinical trials of DPX Survivac or for DPX-SurMAGE, DPX-BRAF or DPX-COVID-19, fail to demonstrate safety and efficacy to the satisfaction of the FDA, Health Canada or similar regulatory authorities outside the United States and Canada or do not otherwise produce positive results, the Corporation may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of the product candidates.
Before obtaining regulatory approval for the sale of any product candidate, the Corporation must conduct extensive clinical trials to demonstrate the safety, purity and potency, or efficacy, of the product candidates in humans. Clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome. A failure of one or more of the Corporation’s clinical trials can occur at any stage of testing. The outcome of preclinical testing and early clinical trials may not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval of their products.
The Corporation may experience numerous unforeseen events during, or as a result of, clinical trials that could delay or prevent the Corporation’s ability to receive regulatory approval or commercialize its product candidates. Unforeseen events that could delay or prevent the Corporation’s ability to receive regulatory approval or commercialize its product candidates include:
S-19
| · | regulators or institutional review boards may not authorize the Corporation or its investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site; |
| · | the Corporation may have delays in reaching or fail to reach agreement on acceptable clinical trial contracts or clinical trial protocols with prospective trial sites; |
| · | clinical trials of the product candidates may produce negative or inconclusive results, and the Corporation may decide, or regulators may require, additional clinical trials be conducted or product development programs be abandoned; |
| · | the number of patients required for clinical trials of the product candidates may be larger than anticipated, enrollment in these clinical trials may be slower than anticipated or participants may drop out of these clinical trials at a higher rate than anticipated; |
| · | the Corporation’s third party contractors may fail to comply with regulatory requirements or meet their contractual obligations in a timely manner, or at all; |
| · | the Corporation might have to suspend or terminate clinical trials of its product candidates for various reasons, including a finding that the participants are being exposed to unacceptable health risks; |
| · | regulators or institutional review boards may require that the Corporation or its investigators suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or a finding that the participants are being exposed to unacceptable health risks; |
| · | the cost of clinical trials of the product candidates may be greater than anticipated; |
| · | the supply or quality of the product candidates or other materials necessary to conduct clinical trials of the product candidates may be insufficient or inadequate; and |
| · | the Corporation’s product candidates may have undesirable side effects or other unexpected characteristics, causing the Corporation or its investigators, regulators or institutional review boards to suspend or terminate the trials. |
In addition, the patients recruited for clinical trials of the product candidates may have a disease profile or other characteristics that are different than expected and different than what the clinical trials were designed for, which could adversely impact the results of the clinical trials.
If the Corporation is required to conduct additional clinical trials or other testing of its product candidates beyond those that are currently contemplated, if the Corporation is unable to successfully complete clinical trials of its product candidates or other testing, if the results of these trials or tests are not positive or are only modestly positive or if there are safety concerns, the Corporation may:
| · | be delayed in obtaining marketing approval for its product candidates; |
| · | not obtain marketing approval at all; |
| · | obtain approval for indications or patient populations that are not as broad as intended or desired; |
| · | obtain approval with labeling that includes significant use restrictions or safety warnings, including boxed warnings; |
| · | have the product removed from the market after obtaining marketing approval; |
| · | be subject to additional post marketing testing requirements; or |
| · | be subject to restrictions on how the product is distributed or used. |
S-20
The Corporation’s product development costs will also increase if delays in testing or approvals are experienced. The Corporation does not know whether any clinical trials will begin as planned, will need to be restructured or will be completed on schedule, or at all. Significant clinical trial delays could also shorten any periods during which the Corporation may have the exclusive right to commercialize its product candidates or allow the Corporation’s competitors to bring products to market before the Corporation does and impair the Corporation’s ability to commercialize its product candidates and may harm the business and results of operations.
If the Corporation experiences delays or difficulties in the enrollment of patients in clinical trials, receipt of necessary regulatory approvals could be delayed or prevented.
The Corporation may not be able to initiate or continue clinical trials for its product candidates, if the Corporation is unable to locate and enroll a sufficient number of eligible patients to participate in these trials as required by the FDA, Health Canada or similar regulatory authorities outside the United States and Canada. In addition, many of the Corporation’s competitors have ongoing clinical trials for product candidates that could be competitive with the Corporation’s product candidates, and patients who would otherwise be eligible for the Corporation’s clinical trials may instead enroll in clinical trials of the Corporation’s competitors’ product candidates.
Patient enrollment is affected by other factors including:
| · | severity of the disease under investigation; |
| · | eligibility criteria for the study in question; |
| · | perceived risks and benefits of the product candidate under study; |
| · | efforts to facilitate timely enrollment in clinical trials; |
| · | patient referral practices of physicians; |
| · | the ability to monitor patients adequately during and after treatment; and |
| · | proximity and availability of clinical trial sites for prospective patients. |
The actual amount of time for full enrollment could be longer than planned. Enrollment delays in these ongoing and planned trials or any of the Corporation’s other clinical trials may result in increased development costs for its product candidates, which would cause the value of the Corporation to decline and limit the Corporation’s ability to obtain additional financing, including financing needed to complete the ongoing and planned trials of DPX Survivac. The Corporation’s inability to enroll a sufficient number of patients for these clinical trials or any of the other clinical trials would result in significant delays or may require the Corporation to abandon one or more clinical trials altogether.
If serious adverse or undesirable side effects are identified during the development of any product candidate, the Corporation may need to abandon or limit the development of some of its product candidates.
All of the Corporation’s product candidates are still in preclinical or clinical development and their risk of failure is high. It is impossible to predict when or if any of the Corporation’s product candidates will prove effective or safe in humans or will receive regulatory approval. If the Corporation’s product candidates are associated with undesirable side effects or have characteristics that are unexpected, the Corporation may need to abandon their development or limit development to certain uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk benefit perspective.
The design or the Corporation’s execution of clinical trials may not support regulatory approval.
The design or execution of a clinical trial can determine whether its results will support regulatory approval and flaws in the design or execution of a clinical trial may not become apparent until the clinical trial is well advanced. In some instances, there can be significant variability in safety or efficacy results between different trials of the same product candidate due to numerous factors, including changes in trial protocols, differences in size and type of the patient populations, adherence to the dosing regimen and other trial protocols and the rate of dropout among clinical trial participants. The Corporation does not know whether any Phase 2, Phase 3 or other clinical trials the Corporation may conduct will demonstrate consistent or adequate efficacy and safety to obtain regulatory approval to market the Corporation’s product candidates.
S-21
Further, the FDA, Health Canada and comparable foreign regulatory authorities have substantial discretion in the approval process and in determining when or whether regulatory approval will be obtained for any of the Corporation’s product candidates. The Corporation’s product candidates may not be approved even if they achieve their primary endpoints in future Phase 3 clinical trials or registration trials. The FDA, Health Canada or other regulatory authorities may disagree with the Corporation’s trial design and the Corporation’s interpretation of data from preclinical studies and clinical trials. In addition, any of these regulatory authorities may change requirements for the approval of a product candidate even after reviewing and providing comments or advice on a protocol for a pivotal Phase 3 clinical trial that has the potential to result in FDA, Health Canada or other agencies’ approval. In addition, any of these regulatory authorities may also approve a product candidate for fewer or more limited indications than the Corporation requests or may grant approval contingent on the performance of costly post-marketing clinical trials. The FDA, Health Canada or other regulatory authorities may not approve the labeling claims that the Corporation believes would be necessary or desirable for the successful commercialization of its product candidates.
Even if any of the Corporation’s product candidates, including DPX Survivac, receive regulatory approval, they may fail to achieve the degree of market acceptance by physicians, patients, healthcare payors and others in the medical community necessary for commercial success.
If DPX Survivac or any other product candidates receive marketing approval, they may nonetheless fail to gain sufficient market acceptance by physicians, patients, healthcare payors and others in the medical community. Gaining market acceptance for the DPX™ based products may be particularly difficult as, to date, the FDA has only approved a limited number of cancer immunotherapies and the DPX™ based products are based on a novel technology. If these products do not achieve an adequate level of acceptance, the Corporation may not generate significant product revenues and may not become profitable. The degree of market acceptance of the Corporation’s product candidates, if approved for commercial sale, will depend on a number of factors, including:
| · | efficacy and potential advantages compared to alternative treatments; |
| · | the ability to offer its product candidates for sale at competitive prices; |
| · | convenience and ease of administration compared to alternative treatments; |
| · | the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; |
| · | the strength of marketing and distribution support; |
| · | sufficient third party coverage or reimbursement; and |
| · | the prevalence and severity of any side effects. |
If the Corporation is unable to establish sales and marketing capabilities or enter into agreements with third parties to sell and market its product candidates, the Corporation may not be successful in commercializing its product candidates if and when they are approved.
The Corporation does not have a sales or marketing infrastructure and has no experience in the sale, marketing or distribution of pharmaceutical products. To achieve commercial success for any of its product that would be approved in the future, the Corporation must either develop a sales and marketing organization or outsource these functions to third parties. The Corporation currently intends to establish commercialization arrangements with third parties.
There are risks involved with entering into arrangements with third parties to perform these services. If the Corporation enters into arrangements with third parties to perform sales, marketing and distribution services, its product revenues or the profitability of these product revenues are likely to be lower than if the Corporation were to market and sell any products that it develops. In addition, the Corporation may not be successful in entering into arrangements with third parties to sell and market its product candidates or doing so on terms that are favorable to the Corporation. The Corporation likely will have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market its products effectively. If the Corporation does not establish sales and marketing capabilities successfully, either on its own or in collaboration with third parties, it will not be successful in commercializing its product candidates.
S-22
The Corporation faces substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than it may.
The development and commercialization of new drug products is highly competitive. The Corporation faces competition with respect to its current or contemplated product candidates, and will face competition with respect to any products that it may seek to develop or commercialize in the future, from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide. There are a number of large pharmaceutical and biotechnology companies that currently market and sell products or are pursuing the development of products for the treatment of the disease indications for which the Corporation is developing its current or contemplated product candidates. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization.
Some of these competitive products and therapies are based on scientific approaches that are the same as or similar to the Corporation’s approaches, and others are based on entirely different approaches. Many marketed therapies for the indications that the Corporation is currently pursuing, or indications that it may in the future seek to address using the DPX platform, are widely accepted by physicians, patients and payors, which may make it difficult for the Corporation to replace with any products that the Corporation successfully develops and are permitted to market.
There are many FDA approved cancer therapies that may provide equivalent or better efficacy compared to DPX Survivac.
In addition, the Corporation estimates that there are numerous cancer immunotherapy products in clinical development by many public and private biotechnology and pharmaceutical companies targeting numerous different cancer types. A number of these are in late stage development. For example, Stimuvax (Merck KGaA), a cancer vaccine in late stage clinical development for the treatment of non small lung cancer (NSLC) may successfully improve overall survival to a better extent than DPX Survivac in the same patient population.
The Corporation’s competitors may develop products that are more effective, safer, more convenient or less costly than any that the Corporation is developing or that would render its product candidates obsolete or non competitive. The Corporation’s competitors may also obtain FDA, Health Canada or other regulatory approval for their products more rapidly than the Corporation.
Many of the Corporation’s competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than the Corporation. Mergers and acquisitions in the pharmaceutical, biotechnology and device industries may result in even more resources being concentrated among a smaller number of the Corporation’s competitors. Smaller and other early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with the Corporation in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, the Corporation’s programs.
Even if the Corporation is able to commercialize any product candidates, the products may become subject to unfavorable pricing regulations, third party reimbursement practices or healthcare reform initiatives, which would harm the business.
The regulations that govern marketing approvals, pricing and reimbursement for new drug products vary widely from country to country. In the United States, recently passed legislation may significantly change the approval requirements in ways that could involve additional costs and cause delays in obtaining approvals. Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. As a result, the Corporation might obtain regulatory approval for a product in a particular country, but then be subject to price regulations that delay the commercial launch of the product, possibly for lengthy time periods, and negatively impact the revenues the Corporation is able to generate from the sale of the product in that country. Adverse pricing limitations may hinder the Corporation’s ability to recoup its investment in one or more product candidates, even if its product candidates obtain regulatory approval.
S-23
The Corporation’s ability to commercialize any products successfully also will depend in part on the extent to which reimbursement for these products and related treatments will be available from government health administration authorities, private health insurers and other organizations. Government authorities and third party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. A primary trend in the United States healthcare industry and elsewhere is cost containment. Government authorities and third party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. Increasingly, third party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. The Corporation cannot be sure that reimbursement will be available for any product that it commercializes and, if reimbursement is available, the level of reimbursement. Reimbursement may impact the demand for, or the price of, any product candidate for which the Corporation obtains marketing approval. Obtaining reimbursement for the Corporation’s products may be particularly difficult because of the higher prices often associated with drugs administered under the supervision of a physician. If reimbursement is not available or is available only to limited levels, the Corporation may not be able to successfully commercialize any product candidate for which the Corporation obtained marketing approval.
There may be significant delays in obtaining reimbursement for newly approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA, Health Canada or similar regulatory authorities outside the United States or Canada. Moreover, eligibility for reimbursement does not imply that any drug will be paid for in all cases or at a rate that covers the Corporation’s costs, including research, development, manufacture, sale and distribution. Interim reimbursement levels for new drugs, if applicable, may also not be sufficient to cover the Corporation’s costs and may not be made permanent. Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used, may be based on reimbursement levels already set for lower cost drugs, and may be incorporated into existing payments for other services. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in Canada or the United States. Third party payors often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement policies. The Corporation’s inability to promptly obtain coverage and profitable payment rates from both government funded and private payors for any approved products that the Corporation develops could have a material adverse effect on the Corporation’s operating results, the Corporation’s ability to raise capital needed to commercialize products and the Corporation’s overall financial condition.
The Corporation’s reliance on government funding adds uncertainty to the Corporation’s research and commercialization efforts of its government funded product candidates.
The Corporation has received significant funding from government organizations since its inception totaling over $15 million. There is no assurance the Corporation will continue to apply for and/or be awarded government funding in the future. If the Corporation is unable to obtain additional government funding, including as it relates to its DPX-COVID-19 program, it will have to either obtain funds through raising additional capital or arrangements with strategic partners or others, if available, that may require the Corporation to relinquish material rights to certain technologies or potential markets. There is no certainty that financing will be available in amounts the Corporation requires to pursue the planned activities or on acceptable terms, if at all.
S-24
Product liability lawsuits against the Corporation could cause the Corporation to incur substantial liabilities and to limit commercialization of any products that the Corporation may develop.
The Corporation faces an inherent risk of product liability exposure related to the testing of its product candidates in human clinical trials and will face an even greater risk if the Corporation commercially sells any products that it may develop. None of the Corporation’s product candidates have been widely used over an extended period of time, and therefore, safety data is limited.
If the Corporation cannot successfully defend itself against claims that its product candidates or products caused injuries, it will incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
| · | decreased demand for any product candidates or products that it may develop; |
| · | injury to the Corporation’s reputation and significant negative media attention; |
| · | withdrawal of clinical trial participants; |
| · | significant costs to defend the related litigation; |
| · | substantial monetary awards to trial participants or patients; |
| · | loss of revenue; and |
| · | the inability to commercialize any products that the Corporation may develop. |
The Corporation currently maintains a clinical trial liability insurance coverage in the amount of $10 million, which may not be adequate to cover all liabilities that it may incur. The Corporation will need to increase its insurance coverage when it begins commercializing its product candidates. Insurance coverage is increasingly expensive. The Corporation may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise.
The Corporation may expend its limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because the Corporation has limited financial and managerial resources, the Corporation focuses on research programs and product candidates for specific indications. As a result, the Corporation may forego or delay pursuit of opportunities with other product candidates or for other indications that later prove to have greater commercial potential. The Corporation’s resource allocation decisions may cause the Corporation to fail to capitalize on viable commercial products or profitable market opportunities. The Corporation’s spending on current and future research and development programs and product candidates for specific indications may not yield any commercially viable products.
The Corporation has based its research and development efforts on its DPX platform. Notwithstanding the large investment to date and anticipated future expenditures in its DPX platform, the Corporation has not yet developed, and may never successfully develop, any marketed drugs using this approach. As a result of pursuing the development of product candidates using the DPX platform, the Corporation may fail to develop product candidates or address indications based on other scientific approaches that may offer greater commercial potential or for which there is a greater likelihood of success.
The Corporation’s long term business plan is to develop DPX™ based products for the treatment of various cancers and infectious diseases. The Corporation may not be successful in its efforts to identify or discover additional product candidates that may be manufactured using its DPX platform. Research programs to identify new product candidates require substantial technical, financial and human resources. These research programs may initially show promise in identifying potential product candidates, yet fail to yield product candidates for clinical development.
If the Corporation does not accurately evaluate the commercial potential or target market for a particular product candidate, the Corporation may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for the Corporation to retain sole development and commercialization rights to such product candidate.
S-25
Risks Related to the Corporation’s Dependence on Third Parties
If the Corporation is not able to establish collaborations, the Corporation may have to alter its development and commercialization plans.
The Corporation’s drug development programs and the potential commercialization of its product candidates will require substantial additional cash to fund expenses. For some of the Corporation’s product candidates, the Corporation plans to collaborate with pharmaceutical and biotechnology companies for the development and potential commercialization of those product candidates.
The Corporation faces significant competition in seeking appropriate collaborators. Whether the Corporation reaches a definitive agreement for a collaboration will depend, among other things, upon its assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration, and the proposed collaborator’s evaluation of a number of factors. Those factors may include the design or results of clinical trials, the likelihood of approval by the FDA, Health Canada or similar regulatory authorities outside the United States and Canada, the potential market for the subject product candidate, the costs and complexities of manufacturing and delivering such product candidate to patients, the potential of competing products, the existence of uncertainty with respect to the Corporation’s ownership of technology, which can exist if there is a challenge to such ownership without regard to the merits of the challenge and industry and market conditions generally. The collaborator may also consider alternative product candidates or technologies for similar indications that may be available to collaborate on and whether such a collaboration could be more attractive than the one with the Corporation for its product candidate. The Corporation may also be restricted under existing license agreements from entering into agreements on certain terms with potential collaborators. Collaborations are complex and time consuming to negotiate and document. The Corporation may not be able to negotiate collaborations on a timely basis, on acceptable terms, or at all.
The Corporation will need to raise capital or develop collaborations with third parties to commercialize its products. If the Corporation is not able to obtain such funding or enter into collaborations for any such product candidate, the Corporation may have to curtail the development of such product candidate, reduce or delay its development program or one or more of its other development programs, delay its potential commercialization or reduce the scope of any sales or marketing activities, or increase its expenditures and undertake development or commercialization activities at the Corporation’s own expense. If the Corporation elects to increase its expenditures to fund development or commercialization activities on its own, the Corporation may need to obtain additional capital, which may not be available to the Corporation on acceptable terms or at all. If the Corporation does not have sufficient funds, the Corporation may not be able to further develop these product candidates or bring these product candidates to market and generate product revenue.
The Corporation expects to depend on collaborations with third parties for the development and commercialization of its product candidates. If those collaborations are not successful, the Corporation may not be able to capitalize on the market potential of these product candidates.
The Corporation intends to establish commercialization arrangements with third parties. The Corporation’s likely collaborators for any development, distribution, marketing, licensing or broader collaboration arrangements include large and mid size pharmaceutical companies, regional and national pharmaceutical companies and biotechnology companies.
Potential delays include delays in manufacture or clinical trials, failure to produce sufficient quantities of product to conduct trials, or failure to complete trials. The Corporation’s collaborators may fail to meet contractual obligations. They could also pursue other technologies or develop alternative products that could compete with the products the Corporation is developing. If the Corporation does enter into any such arrangements with any third parties, the Corporation will likely have limited control over the amount and timing of resources that its collaborators dedicate to the development or commercialization of its product candidates. The Corporation’s ability to generate revenues from these arrangements will depend on its collaborators’ abilities to successfully perform the functions assigned to them in these arrangements.
| S-26 |
Collaborations involving the Corporation’s product candidates would pose the following risks to the Corporation:
| · | collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations; |
| · | collaborators may not pursue development and commercialization of the Corporation’s product candidates or may elect not to continue or renew development or commercialization programs based on clinical trial results, changes in the collaborator’s strategic focus or available funding, or external factors such as an acquisition that diverts resources or creates competing priorities; |
| · | collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing; |
| · | collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with the Corporation’s products or product candidates if the collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive than the Corporation’s; |
| · | a collaborator with marketing and distribution rights to one or more products may not commit sufficient resources to the marketing and distribution of such product or products; |
| · | collaborators may not properly maintain or defend the Corporation’s intellectual property rights or may use the Corporation’s proprietary information in such a way as to invite litigation that could jeopardize or invalidate the Corporation’s proprietary information or expose the Corporation to potential litigation; |
| · | disputes may arise between the collaborators and the Corporation that result in the delay or termination of the research, development or commercialization of the Corporation’s products or product candidates or that result in costly litigation or arbitration that diverts management attention and resources; and |
| · | collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable product candidates. For example, the Corporation could have to build a sales force. |
Collaboration agreements may not lead to development or commercialization of product candidates in the most efficient manner, or at all. In addition, there have been a significant number of recent business combinations among large pharmaceutical companies that have resulted in a reduced number of potential future collaborators. If a present or future collaborator of the Corporation were to be involved in a business combination, the continued pursuit and emphasis on the Corporation’s product development or commercialization program could be delayed, diminished or terminated.
The Corporation relies on third parties to conduct its clinical trials, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such trials.
The Corporation does not independently conduct clinical trials of its product candidates. The Corporation relies on third parties, such as contract research organizations, clinical data management organizations, medical institutions and clinical investigators, to perform this function. The Corporation’s reliance on these third parties for clinical development activities reduces its control over these activities but does not relieve the Corporation of its responsibilities. The Corporation remains responsible for ensuring that each of its clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Moreover, the FDA requires the Corporation to comply with standards, commonly referred to as Good Clinical Practices, for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. The Corporation is also required to register ongoing clinical trials and post the results of completed clinical trials on a government sponsored database, ClinicalTrials.gov, within certain timeframes. Failure to do so can result in fines, adverse publicity and civil and criminal sanctions. Furthermore, these third parties may also have relationships with other entities, some of which may be the Corporation’s competitors. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct the Corporation’s clinical trials in accordance with regulatory requirements or the Corporation’s stated protocols, the Corporation will not be able to obtain, or may be delayed in obtaining, regulatory approvals for its product candidates and will not be able to, or may be delayed in its efforts to, successfully commercialize its product candidates.
| S-27 |
The Corporation also relies on other third parties to store and distribute drug supplies for its clinical trials. Any performance failure on the part of the Corporation’s existing or future distributors could delay clinical development or regulatory approval of its product candidates or commercialization of its products, producing additional losses and depriving the Corporation of potential product revenue.
The Corporation depends on third party suppliers to obtain the Corporation’s raw ingredients and intermediate drug substances, which are necessary for the production of the Corporation’s products.
The Corporation currently procures ingredients and intermediate drug substances for the manufacturing of the Corporation’s pipeline products from specialized suppliers. For some components, including raw ingredients, the Corporation has so far identified only one supplier which is qualified for the Corporation’s GMP process. In the event that a supplier stops supplying the required ingredient(s), the Corporation may need to identify an alternative source of such components and may need to wait until it is qualified for the Corporation’s GMP process before procuring the components, which may cause substantial delays to one or all of the Corporation’s clinical programs.
Risks Related to the Manufacturing of the Corporation’s Product Candidates
Natural disasters, public health crises, political crises, and other catastrophic events outside of our control may damage the facilities or disrupt the operations of our strategic partners, third party manufacturers, suppliers or other third parties upon which we rely, and could delay or impair our ability to initiate or complete our clinical trials or commercialize candidate product.
Our strategic partners, third-party manufacturers, suppliers and other third parties upon which we rely have operations around the world and are exposed to a number of global and regional risks outside of our control. These include, but are not limited to, natural disasters, such as earth quakes, tsunamis, power shortages or outages, floods or monsoons, public health crises, such as pandemics and epidemics, political crises, such as terrorism, war, political instability or other conflict, or other events outside of our control.
In December 2019, a novel strain of coronavirus (COVID-19) emerged in Wuhan, Hubei Province, China. While initially the outbreak was largely concentrated in China and caused significant disruptions to the economy, it has now spread to several other countries and infections have been reported around the world which resulted in the World Health Organization (WHO) declaring a pandemic on March 11, 2020 and have caused governmental authorities and non-governmental entities to introduce measures to try to limit this pandemic. The extent to which coronavirus (COVID-19) impacts our operations will depend on future developments which are highly uncertain and cannot be predicted with confidence. Some components of our products are manufactured by third parties located in other countries, including Germany, Japan and China. The continued spread of the coronavirus (COVID-19) globally could adversely impact our operations, including among others, our manufacturing supply chain, clinical trial operations and could have an adverse impact on our business and financial results.
If the Corporation is unable to commercially manufacture its products, the Corporation could face delayed trial approvals or sales.
The Corporation has no experience manufacturing commercial quantities of products and does not currently have the resources to commercially manufacture any products that the Corporation may develop. Accordingly, if the Corporation becomes successful in developing any product with commercial potential, the Corporation would either be required to develop the facilities to manufacture independently or secure a contract manufacturer or enter into another arrangement with third parties to manufacture such products. If the Corporation is unable to develop such capabilities or enter into any such arrangement on favourable terms, the Corporation may be unable to compete effectively in the marketplace. If the Corporation is unable to manufacture or contract for a sufficient supply of product on acceptable terms, or if the Corporation encounters delays or difficulties in its relationships with manufacturers or collaborators, its preclinical, clinical testing and/or product sales could be delayed, thereby delaying the submission of products for regulatory approval and/or market introduction and subsequent sales of such products.
| S-28 |
Currently the Corporation is utilizing the GMP services of a contract manufacturing organization (“CMO”) located in the United States for its clinical drug product manufacturing and does not have a fully qualified and approved backup facility. The Corporation may need to approve an alternative CMO to avoid delays in planned clinical programs should there be any issues with the current CMO. The Corporation’s products require a unique manufacturing process and uses specialized equipment manufactured by another third party to manufacture the Corporation’s clinical candidate vaccines. The specialized equipment used during the manufacturing process is made by only one manufacturer. In the event of catastrophic equipment failure and in the event that this particular supplier of the equipment ceases its operations and/ or replacement equipment cannot be procured, alternative suppliers of similar equipment may be sought and additional product development may be required, which may cause significant delays to some or all of the Corporation’s clinical programs.
Risks Related to the Corporation’s Intellectual Property
If the Corporation fails to comply with its obligations under its intellectual property licenses with third parties, the Corporation could lose license rights that are important to its business.
The Corporation is a party to a number of intellectual property license agreements with third parties and expects to enter into additional license agreements in the future. The Corporation’s existing license agreements impose, and the Corporation expects that future license agreements will impose, various diligences, milestone payment, royalty, insurance, indemnification and other obligations on the Corporation. If the Corporation fails to comply with its obligations under these licenses, its licensors may have the right to terminate these license agreements, in which event the Corporation might not be able to market any product that is covered by these agreements, or to convert the license to a non-exclusive license, which could materially adversely affect the value of the product candidate being developed under the license agreement. Termination of these license agreements or reduction or elimination of the Corporation’s licensed rights may result in the Corporation having to negotiate new or reinstated licenses with less favorable terms.
If the Corporation is unable to obtain and maintain patent protection for its technology and products, or if the Corporation’s licensors are unable to obtain and maintain patent protection for the technology or products that the Corporation licenses from them, or if the scope of the patent protection obtained is not sufficiently broad, the Corporation’s competitors could develop and commercialize technology and products similar or identical to that of the Corporation’s, and its ability to successfully commercialize its technology and products may be adversely affected.
The Corporation’s success depends in large part on its and its licensors’ ability to obtain and maintain patent protection in the United States and other countries with respect to its proprietary technology and products. The Corporation and its licensors have sought to protect the Corporation’s proprietary position by filing patent applications in the United States and abroad related to its novel technologies and products that are important to its business. This process is expensive and time consuming, and the Corporation may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that the Corporation will fail to identify patentable aspects of its research and development output before it is too late to obtain patent protection. Moreover, in some circumstances, the Corporation does not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the patents, covering technology or products that it licenses from third parties and are reliant on its licensors. Therefore, the Corporation cannot be certain that these patents and applications will be prosecuted and enforced in a manner consistent with the best interests of its business. If such licensors fail to maintain such patents, or lose rights to those patents, the rights the Corporation has licensed may be reduced or eliminated.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of the Corporation’s and its licensors’ patent rights are highly uncertain. The Corporation and its licensors’ pending and future patent applications may not result in patents being issued which protect its technology or products or which effectively prevent others from commercializing competitive technologies and products. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of the Corporation’s patents or narrow the scope of its patent protection.
| S-29 |
The laws of foreign countries may not protect the Corporation’s rights to the same extent as the laws of Canada and the United States. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in Canada and the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, the Corporation cannot be certain that itself or its licensors were the first to make the inventions claimed in its owned or licensed patents or pending patent applications, or that the Corporation or its licensors were the first to file for patent protection of such inventions.
Assuming the other requirements for patentability are met, in the United States, the first to invent the claimed invention is entitled to the patent, while outside the United States, the first to file a patent application is generally entitled to the patent. Under the America Invents Act, or AIA, enacted in September 2011, the United States moved to a first inventor to file system in March 2013. The Corporation may become involved in opposition or interference proceedings challenging its patent rights or the patent rights of others. An adverse determination in any such proceeding or litigation could reduce the scope of, or invalidate, the Corporation’s patent rights, allowing third parties to commercialize its technology or products and compete directly with the Corporation, without payment to the Corporation, or result in its inability to manufacture or commercialize products without infringing third party patent rights. For example, Merck has to maintain patents on antigens licensed to the Corporation.
Even if the Corporation’s owned and licensed patent applications issue as patents, they may not issue in a form that will provide the Corporation with any meaningful protection, prevent competitors from competing with the Corporation or otherwise provide the Corporation with any competitive advantage. The Corporation’s competitors may be able to circumvent its owned or licensed patents by developing similar or alternative technologies or products in a non infringing manner. The issuance of a patent is not conclusive as to its scope, validity or enforceability, and the Corporation’s owned and licensed patents may be challenged in the courts or patent offices in Canada, the United States and abroad. Such challenges may result in patent claims being narrowed, invalidated or held unenforceable, which could limit the Corporation’s ability to or stop or prevent the Corporation from stopping others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of its technology and products. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, the Corporation’s owned and licensed patent portfolio may not provide it with sufficient rights to exclude others from commercializing products similar or identical to the Corporation’s.
The Corporation may become involved in lawsuits to protect or enforce its patents, which could be expensive, time consuming and unsuccessful.
Competitors may infringe the Corporation’s patents. To counter infringement or unauthorized use, the Corporation may be required to file infringement claims, which can be expensive and time consuming. In addition, in an infringement proceeding, a court may decide that a patent of the Corporation’s is invalid or unenforceable or may refuse to stop the other party from using the technology at issue on the grounds that its patents do not cover the technology in question. An adverse result in any litigation proceeding could put one or more of the Corporation’s patents at risk of being invalidated or interpreted narrowly. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of the Corporation’s confidential information could be compromised by disclosure during this type of litigation. In addition, the Corporation’s licensors may have rights to file and prosecute such claims and it is reliant on them.
Third parties may initiate legal proceedings alleging that the Corporation is infringing their intellectual property rights, the outcome of which would be uncertain and could have a material adverse effect on the success of the Corporation’s business.
| S-30 |
The Corporation’s commercial successes depends upon its ability and the ability of its collaborators to develop, manufacture, market and sell its product candidates and use its proprietary technologies without infringing the proprietary rights of third parties. The Corporation may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to its products and technology, including interference proceedings before the U.S. Patent and Trademark Office or other similar regulatory authorities. Third parties may assert infringement claims against the Corporation based on existing patents or patents that may be granted in the future. If the Corporation is found to infringe a third party’s intellectual property rights, it could be required to obtain a license from such third party to continue developing and marketing its products and technology. However, the Corporation may not be able to obtain any required license on commercially reasonable terms or at all. Even if the Corporation was able to obtain a license, it could be non-exclusive, thereby giving its competitors access to the same technologies licensed to the Corporation. The Corporation could be forced, including by court order, to cease commercializing the infringing technology or product. In addition, the Corporation could be found liable for monetary damages. A finding of infringement could prevent the Corporation from commercializing its product candidates or force the Corporation to cease some of its business operations, which could materially harm the Corporation’s business. Claims that the Corporation has misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on its business.
The Corporation has research licenses to certain reagents and their use in the development of its product candidates. The Corporation would need commercial licenses to these reagents for any of the Corporation’s product candidates that receive approval for sale in the United States or Canada. The Corporation believes that commercial licenses to these reagents will be available. If the Corporation is unable to obtain any such commercial licenses, it may be unable to commercialize its product candidates without infringing the patent rights of third parties. If the Corporation did seek to commercialize its product candidates without a license, these third parties could initiate legal proceedings against the Corporation.
The Corporation may be subject to claims that its employees have wrongfully used or disclosed alleged trade secrets of their former employers.
Many of the Corporation’s employees were previously employed at universities or other biotechnology or pharmaceutical companies. Although the Corporation tries to ensure that its employees do not use the proprietary information or know how of others in their work for the Corporation, the Corporation may be subject to claims that it or these employees have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such employee’s former employer. Litigation may be necessary to defend against these claims. If the Corporation fails in defending any such claims, in addition to paying monetary damages, it may lose valuable intellectual property rights or personnel. Even if the Corporation is successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
Intellectual property litigation could cause the Corporation to spend substantial resources and distract its personnel from their normal responsibilities.
Even if resolved in the Corporation’s favor, litigation or other legal proceedings relating to intellectual property claims may cause the Corporation to incur significant expenses, and could distract the Corporation’s technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of the Corporation’s Common Shares. Such litigation or proceedings could substantially increase the Corporation’s operating losses and reduce the resources available for development activities. The Corporation may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of the Corporation’s competitors may be able to sustain the costs of such litigation or proceedings more effectively than it can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on the Corporation’s ability to compete in the marketplace.
If the Corporation is unable to protect the confidentiality of its trade secrets, the Corporation’s business and competitive position would be harmed.
In addition to seeking patents for some of the Corporation’s technology and products, it also relies on trade secrets, including unpatented know how, technology and other proprietary information, to maintain its competitive position. The types of protections available for trade secrets are particularly important with respect to the DPX platform’s manufacturing capabilities, which involve significant unpatented know how. The Corporation seeks to protect these trade secrets, in part, by entering into non disclosure and confidentiality agreements with parties who have access to them, such as the Corporation’s employees, corporate collaborators, outside scientific collaborators, sponsored researchers, contract manufacturers, consultants, advisors and other third parties. The Corporation also enters into confidentiality and invention or patent assignment agreements with its employees and consultants. Despite these efforts, any of these parties may breach the agreements and disclose the Corporation’s proprietary information, including its trade secrets, and the Corporation may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time consuming, and the outcome is unpredictable. In addition, courts in certain jurisdictions are less willing or unwilling to protect trade secrets. If any of the Corporation’s trade secrets were to be lawfully obtained or independently developed by a competitor, it would have no right to prevent them from using that technology or information to compete with the Corporation. If any of the Corporation’s trade secrets were to be disclosed to or independently developed by a competitor, its competitive position would be harmed.
| S-31 |
Cyber security incidents and privacy breaches could result in important remediation costs, increased cyber security costs, litigation and reputational harm.
Cyber security incidents can result from deliberate attacks or unintentional events. Cyber-attacks and security breaches could include unauthorized attempts to access, disable, improperly modify or degrade the Corporation’s information, systems and networks, the introduction of computer viruses and other malicious codes and fraudulent “phishing” emails that seek to misappropriate data and information or install malware onto users’ computers. Cyber-attacks in particular vary in technique and sources, are persistent, frequently change and are increasingly more targeted and difficult to detect and prevent against.
Disruptions due to cyber security incidents could adversely affect the Corporation’s business. In particular, a cyber security incident could result in the loss or corruption of data from the Corporation’s research and development activities, including clinical trials, which may cause significant delays to some or all of the Corporation’s clinical programs. Also, the Corporation’s trade secrets, including unpatented know how, technology and other proprietary information could be disclosed to competitors further to a breach, which would harm the Corporation’s business and competitive position. If the Corporation is unable to protect the confidentiality of its trade secrets, the Corporation’s business and competitive position would be harmed.
The Corporation is subject to privacy and security regulations with respect to the use and disclosure of protected health information. Subject to limited exceptions, the regulations restrict the Corporation’s ability to use or disclose patient identifiable information without patient consent for purposes other than treatment or health-care operations. Any breach of the Corporation’s systems that results in personal information being obtained by unauthorized persons could adversely affect the reputation of the Corporation and lead to litigation, fines and liability for failure to comply with privacy and information security laws.
The Corporation relies on a third-party for its information technology (“IT”) function. The Corporation meets with its third-party IT experts on a bi-annual basis to discuss matters related to cyber security. An IT risk assessment is performed on an annual basis with oversight by the Audit Committee and the functionality of internal controls established as a result of this risk assessment are confirmed with the Corporation’s third-party IT experts on a quarterly basis.
The Corporation must successfully upgrade and maintain its information technology systems.
The Corporation relies on various information technology systems to manage its operations. There are inherent costs and risks associated with maintaining, modifying and/or changing these systems and implementing new systems, including potential disruption of the Corporation’s internal control structure, substantial capital expenditures, additional administration and operating expenses, retention of sufficiently skilled personnel to implement and operate its systems, demands on management time and other risks and costs of delays or difficulties in transitioning to new systems or of integrating new systems into the Corporation’s current systems. In addition, the Corporation’s information technology system implementations may not result in productivity improvements at a level that outweighs the costs of implementation, or at all. The implementation of new information technology systems may also cause disruptions in the Corporation’s business operations and have an adverse effect on its business, prospects, financial condition and operating results.
| S-32 |
Risks Related to Regulatory Approval of the Corporation’s Product Candidates and Other Legal Compliance Matters
If the Corporation is not able to obtain, or if there are delays in obtaining, required regulatory approvals, the Corporation may not be able to commercialize its product candidates, and its ability to generate revenue may be materially impaired.
The Corporation’s product candidates, including DPX Survivac, and the activities associated with their development and commercialization, including their design, testing, manufacture, safety, efficacy, recordkeeping, labeling, storage, approval, advertising, promotion, sale and distribution, are subject to comprehensive regulation by the FDA, Health Canada and by comparable authorities in other countries. Failure to obtain regulatory approval for a product candidate will prevent the Corporation from commercializing the product candidate. The Corporation has not received regulatory approval to market any of its product candidates in any jurisdiction. The Corporation has only limited experience in filing and supporting the applications necessary to gain regulatory approvals and expect to rely on third party contract research organizations to assist it in this process. Securing FDA or Health Canada approval requires the submission of extensive preclinical and clinical data and supporting information to the FDA or Health Canada for each therapeutic indication to establish the product candidate’s safety and efficacy. Securing FDA or Health Canada approval also requires the submission of information about the product manufacturing process to, and inspection of manufacturing facilities by, the FDA or Health Canada. The Corporation’s product candidates may not be effective, may be only moderately effective or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude the Corporation from obtaining regulatory approval or prevent or limit commercial use.
The process of obtaining regulatory approvals, both in the United States and abroad, is expensive, may take many years if additional clinical trials are required, if approval is obtained at all, and can vary substantially based upon a variety of factors, including the type, complexity and novelty of the product candidates involved. To date, the FDA has only approved one active cellular immunotherapy product. Changes in regulatory approval policies during the development period, changes in or the enactment of additional statutes or regulations, or changes in regulatory review for each submitted product application, may cause delays in the approval or rejection of an application. The FDA or Health Canada has substantial discretion in the approval process and may refuse to accept any application or may decide that the Corporation’s data is insufficient for approval and require additional preclinical, clinical or other studies. In addition, varying interpretations of the data obtained from preclinical and clinical testing could delay, limit or prevent regulatory approval of a product candidate. Any regulatory approval the Corporation ultimately obtains may be limited or subject to restrictions or post approval commitments that render the approved product not commercially viable.
If the Corporation experiences delays in obtaining approval or if it fails to obtain approval of its product candidates, the commercial prospects for the Corporation’s product candidates may be harmed and its ability to generate revenues will be materially impaired.
Failure to obtain regulatory approval in international jurisdictions would prevent the Corporation’s product candidates from being marketed abroad.
The Corporation intends to enter into arrangements with third parties under which they would market its products outside Canada or the United States. In order to market and sell the Corporation’s products in the European Union and many other jurisdictions, the Corporation or such third parties must obtain separate regulatory approvals and comply with numerous and varying regulatory requirements. The approval procedure varies among countries and can involve additional testing. The time required to obtain approval may differ substantially from that required to obtain FDA or Health Canada approval. The regulatory approval process outside the United States generally includes all of the risks associated with obtaining FDA or Health Canada approval. In addition, in many countries outside the United States or Canada, it is required that the product be approved for reimbursement before the product can be approved for sale in that country. The Corporation or these third parties may not obtain approvals from regulatory authorities outside the United States or Canada on a timely basis, if at all. Approval by the FDA or Health Canada does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside the United States or Canada does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. The Corporation may not be able to file for regulatory approvals and may not receive necessary approvals to commercialize its products in any market.
| S-33 |
If the Corporation fails to comply with environmental, health and safety laws and regulations, it could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of the Corporation’s business.
The Corporation is subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. The Corporation’s operations involve the use of hazardous and flammable materials, including chemicals and radioactive and biological materials. The Corporation’s operations also produce hazardous waste products. The Corporation generally contract with third parties for the disposal of these materials and wastes. The Corporation cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from the Corporation’s use of hazardous materials, it could be held liable for any resulting damages, and any liability could exceed its resources. The Corporation also could incur significant costs associated with civil or criminal fines and penalties.
Although the Corporation maintains workers’ compensation insurance to cover it for costs and expenses it may incur due to injuries to its employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. The Corporation does not maintain insurance for environmental liability or toxic tort claims that may be asserted against the Corporation in connection with its storage or disposal of biological, hazardous or radioactive materials.
In addition, the Corporation may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair the Corporation’s research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
Any product candidate for which the Corporation obtains marketing approval could be subject to restrictions or withdrawal from the market and the Corporation may be subject to penalties if it fails to comply with regulatory requirements or if it experiences unanticipated problems with its products, when and if any of them are approved.
Any product candidate for which the Corporation obtains marketing approval, along with the manufacturing processes, post approval clinical data, labeling, advertising and promotional activities for such product, will be subject to continual requirements of and review by the FDA and other regulatory authorities. These requirements include, among others, submissions of safety and other post marketing information and reports, registration and listing requirements, cGMP requirements relating to quality control, quality assurance and corresponding maintenance of records and documents, cGTP requirements, requirements regarding the distribution of samples to physicians and recordkeeping. Even if regulatory approval of a product candidate is granted, the approval may be subject to limitations on the indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for costly post marketing testing and surveillance to monitor the safety or efficacy of the product. The FDA closely regulates the post approval marketing and promotion of drugs to ensure drugs are marketed only for the approved indications and in accordance with the provisions of the approved label. The FDA imposes stringent restrictions on manufacturers’ communications regarding off label use and if the Corporation does not market its products for their approved indications, the Corporation may be subject to enforcement action for off label marketing.
In addition, later discovery of previously unknown problems with the Corporation’s products, manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may yield various results, including:
| · | restrictions on such products, manufacturers or manufacturing processes; |
| · | restrictions on the marketing of a product; |
| · | restrictions on product distribution; |
| · | requirements to conduct post marketing clinical trials; |
| · | warning or untitled letters; |
| S-34 |
| · | withdrawal of the products from the market; |
| · | refusal to approve pending applications or supplements to approved applications that it submits; |
| · | recall of products; |
| · | fines, restitution or disgorgement of profits or revenue; |
| · | suspension or withdrawal of regulatory approvals; |
| · | refusal to permit the import or export of the Corporation’s products; |
| · | product seizure; or |
| · | injunctions or the imposition of civil or criminal penalties. |
The Corporation’s future relationships with customers and third party payors will be subject to applicable anti kickback, fraud and abuse and other healthcare laws and regulations, which could expose the Corporation to criminal sanctions, civil penalties, program exclusion, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, physicians and third party payors play a primary role in the recommendation and prescription of any product candidates for which the Corporation obtains marketing approval. The Corporation’s future arrangements with third party payors and customers may expose the Corporation to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which it markets, sells and distributes its products for which it obtains marketing approval. Restrictions under applicable United States federal and state healthcare laws and regulations that may impact the Corporation’s activities, include the following:
| · | the federal healthcare anti kickback statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal and state healthcare programs such as Medicare and Medicaid; |
| · | the federal False Claims Act imposes civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| · | the federal Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| · | the federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of, or payment for, healthcare benefits, items or services; |
| · | the federal transparency requirements under the Health Care Reform Law will require manufacturers of drugs, devices, biologics and medical supplies to report to the Department of Health and Human Services information related to physician payments and other transfers of value and physician ownership and investment interests; and |
| S-35 |
| · | analogous state laws and regulations, such as state anti kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non governmental third party payors, including private insurers, and some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug manufacturers to report information related to payments to physicians and other health care providers or marketing expenditures. |
Efforts to ensure that the Corporation’s business arrangements with third parties will comply with applicable healthcare laws and regulations in each jurisdiction when the Corporation products will eventually be offered will involve substantial costs. It is possible that governmental authorities will conclude that the Corporation’s business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If the Corporation’s operations are found to be in violation of any of these laws or any other governmental regulations that may apply to it, it may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from government funded healthcare programs, such as Medicare and Medicaid in the United States, and the curtailment or restructuring of the Corporation’s operations. If any of the physicians or other providers or entities with whom the Corporation expects to do business are found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Contemporary and future legislation may increase the difficulty and cost for the Corporation to obtain marketing approval of and commercialize its product candidates and affect the prices it may obtain.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of the Corporation’s product candidates, restrict or regulate post approval activities and affect its ability to profitably sell any product candidates for which it obtains marketing approval.
In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (“Medicare Modernization Act”), changed the way Medicare covers and pays for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly and introduced a new reimbursement methodology based on average sales prices for physician administered drugs. In addition, this legislation provided authority for limiting the number of drugs that will be covered in any therapeutic class in certain cases. Cost reduction initiatives and other provisions of this legislation could decrease the coverage and reimbursement that is provided for any approved products. While the Medicare Modernization Act applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates. Therefore, any reduction in reimbursement that results from the Medicare Modernization Act may result in a similar reduction in payments from private payors.
In March 2010, President Obama signed into law the Health Care Reform Law, a law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for health care and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. Effective October 1, 2010, the Health Care Reform Law revises the definition of “average manufacturer price” for reporting purposes, which could increase the amount of Medicaid drug rebates to states. Further, the new law imposes a significant annual fee on companies that manufacture or import branded prescription drug products. Substantial new provisions affecting compliance have also been enacted, which may affect the Corporation’s business practices with health care practitioners. The Corporation will not know the full effects of the Health Care Reform Law until applicable federal and state agencies issue regulations or guidance under the new law. Although it is too early to determine the effect of the Health Care Reform Law, this law appears likely to continue the pressure on pharmaceutical pricing, especially under the Medicare program, and may also increase the Corporation’s regulatory burdens and operating costs.
Since its enactment, there have been judicial and Congressional challenges to certain aspects of the Health Care Reform Law. As a result, there have been delays in the implementation of, and action taken to repeal or replace, certain aspects of the Health Care Reform Law. The Corporation expects that the current Presidential Administration and U.S. Congress will likely continue to seek to modify, repeal, or otherwise invalidate all, or certain provisions of, the Health Care Reform Law. The Corporation cannot be sure whether legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of the Corporation’s product candidates, if any, may be.
| S-36 |
With the enactment of the Biologics Price Competition and Innovation Act of 2009 (“BPCIA”), as part of the Health Care Reform Law, an abbreviated pathway for the approval of biosimilar and interchangeable biological products was created. The new abbreviated regulatory pathway establishes legal authority for the FDA to review and approve biosimilar biologics, including the possible designation of a biosimilar as “interchangeable” based on its similarity to an existing brand product. Under the BPCIA, an application for a biosimilar product cannot be submitted to the FDA until four years, or approved by the FDA until 12 years, after the original brand product identified as the reference product was approved under a BLA. The BPCIA is complex and is only beginning to be interpreted and implemented by the FDA. As a result, its ultimate impact, implementation and meaning is subject to uncertainty. While it is uncertain when any such processes may be fully adopted by the FDA, any such processes could have a material adverse effect on the future commercial prospects for the Corporation’s biological products.
The Corporation believes that if any of its product candidates were to be approved as biological products under a BLA, such approved products should qualify for the four year and 12 year periods of exclusivity. However, there is a risk that the United States Congress could amend the BPCIA to significantly shorten these exclusivity periods, or that the FDA will not consider the Corporation’s product candidates to be reference products for competing products, potentially creating the opportunity for generic competition sooner than anticipated. Moreover, the extent to which a biosimilar, once approved, will be substituted for any one of the Corporation’s reference products in a way that is similar to traditional generic substitution for non biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing.
General Company-Related Risks
The Corporation’s future success depends on its ability to retain its key executives and to attract, retain and motivate qualified personnel.
The Corporation is highly dependent on its executive officers. Although the Corporation has formal employment agreements with each of its executive officers, these agreements do not prevent the Corporation’s executives from terminating their employment with the Corporation at any time. The loss of the services of any of these persons could impede the achievement of the Corporation’s research, development and commercialization objectives.
Recruiting and retaining qualified scientific, clinical, manufacturing and sales and marketing personnel will also be critical to the Corporation’s success. The Corporation may not be able to attract and retain these personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. The Corporation also experiences competition for the hiring of scientific and clinical personnel from universities and research institutions. In addition, the Corporation relies on consultants and advisors, including scientific and clinical advisors, to assist it in formulating its research and development and commercialization strategy. The Corporation’s consultants and advisors may be employed by employers other than the Corporation and may have commitments under consulting or advisory contracts with other entities that may limit their availability to the Corporation.
The Corporation may be unable to obtain scientific research and experimental development tax incentive credits.
The Corporation is eligible for scientific research and experimental development tax incentive credits in Canada. There is a risk that a Canadian federal or provincial governmental agency could conclude that: (i) some or all of the expenditures were not incurred on scientific research and experimental development activities, (ii) the rate applicable to such credit is different from the rate claimed by the Corporation, and (iii) the related entity does not meet specified criteria for refundable tax credits, and therefore the governmental agency could reduce or disallow claims for such credits, including refundable credits previously funded. Furthermore, if the Canadian taxation authorities reduce the tax credit either by reducing the rate of the credit or the eligibility of some research and development expenses in the future, our operating results will be materially adversely affected.
| S-37 |
The Corporation expects to expand its development, regulatory, manufacturing and sales and marketing capabilities, and as a result, the Corporation may encounter difficulties in managing its growth, which could disrupt the Corporation’s operations.
The Corporation expects to experience significant growth in the number of its employees and the scope of its operations, particularly in the areas of drug development, regulatory affairs, manufacturing and sales and marketing. To manage the Corporation’s anticipated future growth, it must continue to implement and improve its managerial, operational and financial systems, expand its facilities and continue to recruit and train additional qualified personnel. Due to the Corporation’s limited financial resources, the Corporation may not be able to effectively manage the expansion of its operations or recruit and train additional qualified personnel. The physical expansion of the Corporation’s operations may lead to significant costs and may divert its management and business development resources. Any inability to manage growth could delay the execution of the Corporation’s business plans or disrupt the Corporation’s operations.
The Corporation may acquire businesses or products, or form strategic alliances, in the future, and the Corporation may not realize the benefits of such acquisitions.
The Corporation may acquire additional businesses or products, form strategic alliances or create joint ventures with third parties that the Corporation believes will complement or augment its existing business. If the Corporation acquires businesses with promising products or technologies, the Corporation may not be able to realize the benefit of acquiring such businesses if the Corporation is unable to successfully integrate them with its existing operations and company culture. The Corporation may encounter numerous difficulties in developing, manufacturing and marketing any new products resulting from a strategic alliance or acquisition that delay or prevent it from realizing their expected benefits or enhancing the Corporation’s business. The Corporation cannot assure investors that, following any such acquisition, it will achieve the expected synergies to justify the transaction.
The Corporation has limited experience operating internationally, is subject to a number of risks associated with its international activities and operations, and may not be successful in its efforts to expand internationally.
The Corporation currently has very limited operations outside of Canada. In order to meet the Corporation’s long-term goals, the Corporation would need to grow its international operations significantly. Consequently, the Corporation is and will continue to be subject to additional risks related to operating in foreign countries, including:
| · | the fact that the Corporation has limited experience operating its business internationally; |
| · | local, economic and political conditions, including inflation, geopolitical events, such as war and terrorism, foreign currency fluctuations and exchange risks, which could result in increased or unpredictable operating expenses and reduced revenues and other obligations incident to doing business in, or with a company located in, another country; |
| · | the Corporation’s customers’ ability to obtain reimbursement for any product candidate in foreign markets, and unexpected changes in reimbursement and pricing requirements, tariffs, trade barriers and regulatory requirements; |
| · | different medical practices and customs in foreign countries affecting acceptance in the marketplace; |
| · | longer lead times for shipping and longer accounts receivable collection times; |
| · | the interpretation of contractual provisions governed by foreign laws in the event of a contract dispute; |
| · | reduced protection of intellectual property rights in some foreign countries or the existence of additional potentially relevant third party intellectual property rights; and |
| · | compliance with foreign laws, rules and regulations, including data privacy requirements, labor relations laws, tax laws, accounting requirements, anti-competition regulations, import, export and trade restrictions, anti-bribery/anti-corruption laws, regulations or rules, which could lead to actions by the Corporation or its licensees, distributors, manufacturers, other third parties who act on its behalf or with whom the Corporation does business in foreign countries or the Corporation’s employees who are working abroad that could subject the Corporation to investigation or prosecution under such foreign laws. |
S-38
If we are classified as a passive foreign investment company (“PFIC”) for United States federal income tax purposes, as we expect to be, certain adverse tax rules may apply to U.S. Holders of the Common Shares.
Based on the composition of the Corporation’s income and the value of its assets, the Corporation believes that it is a PFIC for United States federal income tax purposes for the 2019 taxable year and, based on estimates of the Corporation's income and assets for 2020, the Corporation believes that it is likely to be a PFIC for the 2020 taxable year.
The Corporation will be classified as a PFIC for any taxable year for United States federal income tax purposes if either (i) 75% or more of its gross income in that taxable year is passive income or (ii) the average percentage of its assets by value in that taxable year which produce or are held for the production of passive income (which includes cash) is at least 50%.
PFIC status is determined annually and depends upon the composition of a company’s income and assets and the market value of its stock from time to time. Therefore, there can be no assurance as to the Corporation’s PFIC status for future taxable years. The value of the Corporation’s assets will be based, in part, on the then market value of its Common Shares, which is subject to change.
If the Corporation is a PFIC for any taxable year during which a U.S. Holder (as defined under “Certain U.S. Federal Income Tax Considerations” in this prospectus) holds Common Shares, such U.S. Holders could be subject to adverse United States federal income tax consequences whether or not the Corporation continues to be a PFIC. For example, U.S. Holders may become subject to increased tax liabilities under United States federal income tax laws and regulations, and will become subject to burdensome reporting requirements. If the Corporation is a PFIC during a taxable year in which a U.S. Holder holds Common Shares, such U.S. Holder may be able to make a “mark-to-market” election or a “qualified electing fund” election that could mitigate the adverse United States federal income tax consequences that would otherwise apply to such U.S. Holder. Although upon request of a U.S. Holder, the Corporation will provide the information necessary for a U.S. Holder to make the qualified electing fund election with respect to the Corporation, no assurance can be given that such information will be available for any lower-tier PFIC that the Corporation does not control. See “Certain U.S. Federal Income Tax Considerations” for additional information.
U.S. Holders are urged to consult their own tax advisers as to the United State federal income tax consequences related to the Corporation’s expected classification as a PFIC.
United States investors may not be able to obtain enforcement of civil liabilities against the Corporation.
The enforcement by investors of civil liabilities under the United States federal or state securities laws may be affected adversely by the fact that the Corporation is governed by the Canada Business Corporations Act, that the majority of the Corporation officers and directors are residents of Canada, and that all, or a substantial portion of their assets and a substantial portion of the Corporation assets, are located outside the United States. It may not be possible for investors to effect service of process within the United States on certain of its directors and officers or enforce judgments obtained in the United States courts against the Corporation or certain of the Corporation directors and officers based upon the civil liability provisions of United States federal securities laws or the securities laws of any state of the United States.
As a foreign private issuer, the Corporation is subject to different U.S. securities laws and rules than a domestic U.S. issuer, which may limit the information publicly available to its U.S. shareholders.
The Corporation is a foreign private issuer under applicable U.S. federal securities laws and, therefore, is not required to comply with all of the periodic disclosure and current reporting requirements of the United States Securities Exchange Act of 1934, as amended (the “Exchange Act”) and related rules and regulations. As a result, the Corporation does not file the same reports that a U.S. domestic issuer would file with the United States Securities and Exchange Commission (the “SEC”), although it will be required to file with or furnish to the SEC the continuous disclosure documents that the Corporation is required to file in Canada under Canadian securities laws. In addition, the Corporation’s officers, directors and principal shareholders are exempt from the reporting and “short swing” profit recovery provisions of Section 16 of the Exchange Act. Therefore, the Corporation’s shareholders may not know on as timely a basis when its officers, directors and principal shareholders purchase or sell securities of IMV as the reporting periods under the corresponding Canadian insider reporting requirements are longer. In addition, as a foreign private issuer, the Corporation is exempt from the proxy rules under the Exchange Act.
S-39
The Corporation may lose its foreign private issuer status in the future, which could result in significant additional costs and expenses to the Corporation.
In order to maintain its current status as a foreign private issuer, a majority of the Corporation’s Common Shares must be either directly or indirectly owned of record by non-residents of the United States unless the Corporation also satisfies one of the additional requirements necessary to preserve this status. The Corporation may in the future lose its foreign private issuer status if a majority of the Common Shares are owned of record in the United States and the Corporation fails to meet the additional requirements necessary to avoid loss of foreign private issuer status. The regulatory and compliance costs to the Corporation under U.S. federal securities laws as a U.S. domestic issuer may be significantly more than the costs the Corporation incurs as a Canadian foreign private issuer eligible to use the multijurisdictional disclosure system (“MJDS”). If the Corporation is not a foreign private issuer, it would not be eligible to use the MJDS or other foreign issuer forms and would be required to file periodic and current reports and registration statements on U.S. domestic issuer forms with the SEC, which are more detailed and extensive than the forms available to a foreign private issuer. In addition, the Corporation may lose the ability to rely upon exemptions from NASDAQ corporate governance requirements that are available to foreign private issuers.
Risks Relating to this Offering
Management will have broad discretion as to the use of the proceeds from the Offering and may not use the proceeds effectively.
Management of the Corporation will have broad discretion in the application of the net proceeds from the Offering and could spend the proceeds in ways that do not improve the results of operations of the Corporation or enhance the value of the Common Shares. Failure to apply these funds effectively could have a material adverse effect on the business of the Corporation, delay the development of its product candidates, and cause the price of the Common Shares to decline. There is no minimum amount of funds that must be raised under this offering. This means that the Corporation could complete this offering after raising only a small proportion of the proposed offering amount.
The market price of the Common Shares has been and is likely to continue to be volatile and an investment in Common Shares may suffer a decline in value.
You should consider an investment in Common Shares as risky and invest only if you can withstand a significant loss and wide fluctuations in the market value of your investment. The Corporation receives only limited attention by securities analysts and frequently experiences an imbalance between supply and demand for Common Shares. The market price of the Common Shares has been highly volatile and is likely to continue to be volatile. This leads to a heightened risk of securities litigation pertaining to such volatility. Factors such as the financial position of the Corporation and the ability of the Corporation to continue as a going concern; the ability to raise additional capital; the progress of the clinical trials; the ability to obtain partners and collaborators to assist with the future development of the products; general market conditions; announcements of technological innovations or new product candidates by the Corporation, the Corporation collaborators or its competitors; published reports by securities analysts; developments in patent or other intellectual property rights; public concern as to the safety and efficacy of drugs that the Corporation and its competitors develop; and shareholder interest in the Common Shares all contribute to the volatility of the market price of the Common Shares.
S-40
Future sales of Common Shares by the Corporation or by its existing shareholders could cause the market price of the Common Shares to fall.
The issuance of Common Shares by the Corporation could result in significant dilution in the equity interest of existing shareholders and adversely affect the market price of the Common Shares. Sales by existing shareholders of a large number of Common Shares in the public market and the issuance of shares issued in connection with strategic alliances, or the perception that such additional sales could occur, could cause the market price of the Common Shares to decline and have an undesirable impact on the Corporation’s ability to raise capital. With any additional sale or issuance of Common Shares, investors will suffer dilution to their voting power and the Corporation may experience dilution in its earnings per Common Share.
Dilution of purchasers.
Purchasers who purchase Offered Shares as part of the Offering may pay more for the Offered Shares than the amounts paid by existing shareholders or security holders of the Corporation for their Common Shares. As a result, such purchasers may incur immediate and substantial dilution. Convertible securities have been issued and may be issued in the future by the Corporation at a lower price than the current market value of the Common Shares, consequently, purchasers who purchase Offered Shares under the Offering may incur substantial dilution in the near future.
There is no assurance of a sufficient liquid trading market for the Common Shares in the future.
Shareholders of the Corporation may be unable to sell significant quantities of Common Shares into the public trading markets without a significant reduction in the price of their Common Shares, or at all. There can be no assurance that there will be sufficient liquidity of the Common Shares on the trading market, and that the Corporation will continue to meet the listing requirements of the TSX or Nasdaq or achieve listing on any other public listing exchange.
No dividends have been paid on the Common Shares and the Corporation does not intend to pay dividends in the foreseeable future although it may ultimately do so in the appropriate circumstances.
The Corporation has paid no cash dividends on any of its Common Shares to date and currently intends to retain its future earnings, if any, to fund the development growth of its businesses. In addition, the terms of any future debt or credit facility may preclude the Corporation from paying any dividends unless certain consents are obtained and certain conditions are met.
As a passive foreign investment company (“PFIC”) for United States federal income tax purposes, certain adverse tax rules may apply to U.S. Holders of the Common Shares.
Based on the composition of the Corporation’s income and the value of its assets, the Corporation believes that it is a PFIC for United States federal income tax purposes for the 2019 taxable year and, based on estimates of the Corporation’s income and assets for 2020, the Corporation believes that it is likely to be a PFIC for the 2020 taxable year.
The Corporation will be classified as a PFIC for any taxable year for United States federal income tax purposes if either (i) 75% or more of its gross income in that taxable year is passive income or (ii) the average percentage of its assets by value in that taxable year which produce or are held for the production of passive income (which includes cash) is at least 50%.
PFIC status is determined annually and depends upon the composition of a company’s income and assets and the market value of its stock from time to time. Therefore, there can be no assurance as to the Corporation’s PFIC status for future taxable years. The value of the Corporation’s assets will be based, in part, on the then market value of its Common Shares, which is subject to change.
If the Corporation is a PFIC for any taxable year during which a U.S. Holder (as defined under “Certain U.S. Federal Income Tax Considerations” in this prospectus) holds Common Shares, such U.S. Holders could be subject to adverse United States federal income tax consequences whether or not the Corporation continues to be a PFIC. For example, U.S. Holders may become subject to increased tax liabilities under United States federal income tax laws and regulations, and will become subject to burdensome reporting requirements. If the Corporation is a PFIC during a taxable year which a U.S. Holder holds Common Shares, such U.S. Holder may be able to make a “mark-to-market” election or a “qualified electing fund” election that could mitigate the adverse United States federal income tax consequences that would otherwise apply to such U.S. Holder. Although upon request of a U.S. Holder, the Corporation will provide the information necessary for a U.S. Holder to make the qualified electing fund election, no assurance can be given that such information will be available for any lower-tier PFIC that the Corporation does not control. See “Certain U.S. Federal Income Tax Considerations” for additional information.
S-41
U.S. Holders are urged to consult their own tax advisers as to the United State federal income tax consequences related to the Corporation’s classification as a PFIC
Certain Canadian Federal Income Tax Considerations
In the opinion of McCarthy Tétrault LLP, counsel to the Corporation, the following is, as of the date of this prospectus supplement, a summary of the principal Canadian federal income tax considerations under the Income Tax Act (Canada) (“Tax Act”) and the regulations thereunder generally applicable to an investor who acquires as beneficial owner Offered Shares pursuant to the Offering and who, for the purposes of the Tax Act and at all relevant times deals at arm’s length with the Corporation and Piper Sandler, is not affiliated with the Corporation or Piper Sandler (a “Holder”).
The following section of this summary is generally applicable to Holders who for the purposes of the Tax Act and any applicable income tax treaty and at all relevant times, (i) are neither resident nor deemed to be resident in Canada and (ii) do not use or hold, and will not be deemed to use or hold, the Offered Shares in connection with, or in the course of carrying on, a business in Canada (“Non-Resident Holders”). This summary does not apply to a Non-Resident Holder that is (i) an insurer carrying on business in Canada and elsewhere or (ii) that is an “authorized foreign bank” (as defined in the Tax Act). Such Non-Resident Holders should consult their own tax advisors.
This summary is based upon the current provisions of the Tax Act and the regulations thereunder in force as of the date hereof (the “Regulations”) and counsel’s understanding of the administrative policies and assessing practices of the Canada Revenue Agency (the “CRA”) published in writing by the CRA prior to the date hereof. This summary takes into account all specific proposals to amend the Tax Act and the Regulations publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date hereof (the “Tax Proposals”) and assumes that the Tax Proposals will be enacted in the form proposed, although no assurance can be given that the Tax Proposals will be enacted in their current form or at all. Other than the Tax Proposals, this summary does not otherwise take into account or anticipate any changes in law, whether by legislative, governmental, administrative or judicial decision or action, nor does it take into account or consider any provincial, territorial or foreign income tax considerations, which considerations may differ significantly from the Canadian federal income tax considerations discussed in this summary. This summary also does not take into account any change in the administrative policies or assessing practices of the CRA.
This summary is of a general nature only, is not exhaustive of all possible Canadian federal income tax considerations and is not intended to be, nor should it be construed to be, legal or tax advice to any particular Holder. Holders should consult their own tax advisors with respect to their particular circumstances.
Currency
For purposes of the Tax Act, all amounts relating to the acquisition, holding or disposition of the Offered Shares (including dividends, adjusted cost base and proceeds of disposition) must be expressed in Canadian dollars based on the rate as quoted by the Bank of Canada for the applicable day or such other rate of exchange that is acceptable to the CRA.
S-42
Dividends
Dividends paid or credited or deemed to be paid or credited to a Non-Resident Holder by the Corporation are subject to Canadian withholding tax at the rate of 25% on the gross amount of the dividend unless such rate is reduced by the terms of an applicable tax treaty. For example, under the Canada-United States Tax Convention (1980), as amended (the “Treaty”), the rate of withholding tax on dividends paid or credited to a Non-Resident Holder beneficially owning the dividends who is resident in the U.S. for purposes of the Treaty and who is fully entitled to the benefits of the Treaty (a “U.S. Holder”) is generally limited to 15% of the gross amount of the dividend (or 5% in the case of a U.S. Holder that is a corporation beneficially owning at least 10% of the Corporation’s voting shares). Non-Resident Holders are urged to consult their own tax advisors to determine their entitlement to relief under an applicable income tax treaty.
Dispositions of Offered Shares
Upon a disposition (or a deemed disposition) of an Offered Share (other than to the Corporation unless purchased by the Corporation in the open market in the manner in which shares are normally purchased by any member of the public in the open market), a Non-Resident Holder generally will realize a capital gain (or a capital loss) equal to the amount by which the proceeds of disposition of such security, as applicable, net of any reasonable costs of disposition, are greater (or are less) than the adjusted cost base of such security to the Non-Resident Holder.
A Non-Resident Holder generally will not be subject to tax under the Tax Act in respect of a capital gain realized on the disposition or deemed disposition of an Offered Share (other than to the Corporation unless purchased by the Corporation in the open market in the manner in which shares are normally purchased by any member of the public in the open market), unless the Offered Share constitutes “taxable Canadian property” to the Non-Resident Holder thereof for purposes of the Tax Act, and the Non-Resident Holder is not entitled to relief under the terms of an applicable tax treaty. In addition, capital losses arising on the disposition or deemed disposition of an Offered Share will not be recognized under the Tax Act, unless the Offered Share constitutes “taxable Canadian property” to the Non-Resident Holder thereof for purposes of the Tax Act, and the Non-Resident Holder is not entitled to relief under the terms of an applicable tax treaty.
Provided the Common Shares are listed on a “designated stock exchange”, as defined in the Tax Act (which currently includes the NASDAQ and the TSX), at the time of disposition, the Offered Shares generally will not constitute taxable Canadian property of a Non-Resident Holder at that time, unless at any time during the 60 month period immediately preceding the disposition the following two conditions are met concurrently: (i) one or any combination of (a) the Non-Resident Holder, (b) persons with whom the Non-Resident Holder did not deal at arm’s length, and (c) partnerships in which the Non-Resident Holder or a person with whom the Non-Resident Holder did not deal at arm’s length held a membership interest directly or indirectly through one or more partnerships owned 25% or more of the issued shares of any class or series of shares of the Corporation; and (ii) more than 50% of the fair market value of the Common Shares of the Corporation was derived directly or indirectly from one or any combination of (a) real or immovable property situated in Canada, (b) “Canadian resource properties” (as defined in the Tax Act), (c) “timber resource properties” (as defined in the Tax Act) and (d) an option in respect of, an interest in, or for civil law rights in, any of the foregoing property, whether or not such property exists. Notwithstanding the foregoing, an Offered Share may in certain circumstances otherwise be deemed to be taxable Canadian property to a Non-Resident Holder for purposes of the Tax Act.
Non-Resident Holders whose Offered Shares are taxable Canadian property should consult their own tax advisors.
Certain U.S. Federal Income Tax Considerations
The following is a general summary of certain material U.S. federal income tax considerations relevant to a U.S. Holder (as defined below) arising from and relating to the acquisition, ownership, and disposition of Common Shares acquired pursuant to this Prospectus.
This summary is for general information purposes only and does not purport to be a complete analysis or listing of all potential U.S. federal income tax consequences that may apply to a U.S. Holder arising from and relating to the acquisition, ownership, and disposition of Common Shares. In addition, this summary does not take into account the individual facts and circumstances of any particular U.S. Holder that may affect the U.S. federal income tax consequences to such U.S. Holder. Accordingly, this summary is not intended to be, and should not be construed as, legal or U.S. federal income tax advice with respect to any U.S. Holder. Except as discussed below, this summary does not discuss applicable income tax reporting requirements. This summary does not address the U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences to U.S. Holders of the acquisition, ownership, and disposition of Common Shares. Each prospective U.S. Holder should consult its own tax advisor regarding the U.S. federal, U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences relating to the acquisition, ownership, and disposition of Common Shares.
S-43
No legal opinion from U.S. legal counsel or ruling from the Internal Revenue Service (the “IRS”) has been requested, or will be obtained, regarding the U.S. federal income tax consequences of the acquisition, ownership, and disposition of Common Shares. This summary is not binding on the IRS, and the IRS is not precluded from taking a position that is different from, and contrary to, the positions taken in this summary. In addition, because the authorities upon which this summary is based are subject to various interpretations, the IRS and the U.S. courts could disagree with one or more of the positions taken in this summary.
Scope of this Summary
Authorities
This summary is based on the Internal Revenue Code of 1986, as amended (the “Code”), Treasury Regulations (whether final, temporary, or proposed), published rulings of the IRS, published administrative positions of the IRS, the Convention Between Canada and the United States of America with Respect to Taxes on Income and on Capital, signed September 26, 1980, as amended (the “Canada-U.S. Tax Convention”), and U.S. court decisions that are applicable and, in each case, as in effect and available, as of the date of this document. Any of the authorities on which this summary is based could be changed in a material and adverse manner at any time, and any such change could be applied on a retroactive or prospective basis. This summary does not discuss the potential effects, whether adverse or beneficial, of any proposed legislation that, if enacted, could be applied on a retroactive or prospective basis.
U.S. Holders
For purposes of this summary, the term “U.S. Holder” means a beneficial owner of Common Shares acquired pursuant to this Prospectus that is for U.S. federal income tax purposes:
| - | an individual who is a citizen or resident of the U.S.; |
| - | a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) organized under the laws of the U.S., any state thereof or the District of Columbia; |
| - | an estate whose income is subject to U.S. federal income taxation regardless of its source; or |
| - | a trust that (a) is subject to the primary supervision of a court within the U.S. and one or more U.S. persons has the authority to make all substantial decisions of the trust or (b) has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person. |
Non-U.S. Holders
For purposes of this summary, a “non-U.S. Holder” is a beneficial owner of Common Shares that is not a U.S. Holder and is not treated as a partnership for U.S. federal income tax purposes. This summary does not address the U.S. federal income tax consequences to non-U.S. Holders arising from and relating to the acquisition, ownership, and disposition of Common Shares. Accordingly, a non-U.S. Holder should consult its own tax advisor regarding the U.S. federal, U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and foreign tax consequences (including the potential application of and operation of any income tax treaties) relating to the acquisition, ownership, and disposition of Common Shares.
S-44
U.S. Holders Subject to Special U.S. Federal Income Tax Rules Not Addressed
This summary does not address the U.S. federal income tax considerations applicable to U.S. Holders that are subject to special provisions under the Code, including, but not limited to, the following: (a) U.S. Holders that are tax-exempt organizations, qualified retirement plans, individual retirement accounts, or other tax-deferred accounts; (b) U.S. Holders that are financial institutions, underwriters, insurance companies, real estate investment trusts, or regulated investment companies; (c) U.S. Holders that are broker-dealers, dealers, or traders in securities or currencies that elect to apply a mark-to-market accounting method; (d) U.S. Holders that have a “functional currency” other than the U.S. dollar; (e) U.S. Holders that own Common Shares as part of a straddle, hedging transaction, conversion transaction, constructive sale, or other arrangement involving more than one position; (f) U.S. Holders that acquired Common Shares in connection with the exercise of employee stock options or otherwise as compensation for services; (g) U.S. Holders that hold Common Shares other than as a capital asset within the meaning of Section 1221 of the Code (generally, property held for investment purposes); or (h) U.S. Holders that own, have owned or will own (directly, indirectly, or by attribution) 10% or more of the total combined voting power or value of the outstanding Common Shares of the Corporation. This summary also does not address the U.S. federal income tax considerations applicable to U.S. Holders who are: (a) U.S. expatriates or former long-term residents of the U.S.; (b) persons that have been, are, or will be a resident or deemed to be a resident in Canada for purposes of the Tax Act; (c) persons that use or hold, will use or hold, or that are or will be deemed to use or hold Common Shares in connection with carrying on a business in Canada; (d) persons whose Common Shares constitute “taxable Canadian property” under the Tax Act; or (e) persons that have a permanent establishment in Canada for the purposes of the Canada-U.S. Tax Convention. U.S. Holders that are subject to special provisions under the Code, including, but not limited to, U.S. Holders described immediately above, should consult their own tax advisor regarding the U.S. federal, U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences relating to the acquisition, ownership and disposition of Common Shares.
If an entity or arrangement that is classified as a partnership for U.S. federal income tax purposes holds Common Shares, the U.S. federal income tax consequences to such entity and the partners (or other owners) of such partnership generally will depend on the activities of the partnership and the status of such partners. This summary does not address the tax consequences to any such owner. Partners of entities or arrangements that are classified as partnerships for U.S. federal income tax purposes should consult their own tax advisors regarding the U.S. federal income tax consequences arising from and relating to the acquisition, ownership, and disposition of Common Shares.
Ownership and Disposition of Common Shares
The following discussion is subject in its entirety to the rules described below under the heading “Passive Foreign Investment Company Rules”.
Taxation of Distributions
A U.S. Holder that receives a distribution, including a constructive distribution, with respect to a Common Share will be required to include the gross amount of such distribution in income as a dividend (without reduction for any Canadian or foreign income tax withheld from such distribution) to the extent of the U.S. Holder’s pro rata share of the current or accumulated “earnings and profits” of the Corporation, as computed for U.S. federal income tax purposes. To the extent that a distribution exceeds the U.S. Holder’s pro rata share of the current and accumulated “earnings and profits” of the Corporation, such distribution will be treated first as a tax-free return of capital to the extent of a U.S. Holder’s tax basis in the Common Shares and thereafter as gain from the sale or exchange of such Common Shares (see “Sale or Other Taxable Disposition of Common Shares”). However, the Corporation may not maintain the calculations of earnings and profits in accordance with U.S. federal income tax principles, and each U.S. Holder should therefore assume that any distribution by the Corporation with respect to the Common Shares will constitute ordinary dividend income. Dividends received on Common Shares generally will not be eligible for the “dividends received deduction” available to U.S. corporate shareholders receiving dividends from U.S. corporations.
Subject to applicable limitations and provided the Corporation is eligible for the benefits of the Canada – U.S. Tax Convention or the Common Shares are readily tradable on a United States securities market, dividends paid by the Corporation to non-corporate U.S. Holders, including individuals, generally will be eligible for the preferential tax rates applicable to long-term capital gains for dividends provided certain holding period and other conditions are satisfied, including that the Corporation not be classified as a PFIC in the tax year of distribution or in the preceding tax year. The dividend rules are complex, and each U.S. Holder should consult its own tax advisor regarding the application of such rules.
S-45
Sale or Other Taxable Disposition of Common Shares
A U.S. Holder will recognize gain or loss on the sale or other taxable disposition of Common Shares in an amount equal to the difference, if any, between (a) the amount of cash plus the fair market value of any property received on the sale or other taxable disposition and (b) such U.S. Holder’s tax basis in such Common Shares sold or otherwise disposed of. Any such gain or loss generally will be capital gain or loss, which will be long-term capital gain or loss if, at the time of the sale or other disposition, such Common Shares are held for more than one year.
Preferential tax rates apply to long-term capital gains of non-corporate U.S. Holders. There are currently no preferential tax rates for long-term capital gains of a U.S. Holder that is a corporation. Deductions for capital losses are subject to significant limitations under the Code. A U.S. Holder’s tax basis in Common Shares generally will be such U.S. Holder’s U.S. dollar cost for such Common Shares.
Passive Foreign Investment Company
Based on the projected composition of the Corporation’s income, the Corporation believes that it is a PFIC for United States federal income tax purposes for the 2019 taxable year and that it is likely to be a PFIC for the 2020 taxable year. The determination of whether the Corporation will continue as a PFIC is made annually.
In general, the Corporation will be a PFIC for any taxable year in which:
| · | at least 75% of the Corporation’s gross income is passive income, or |
| · | at least 50% of the value (determined based on a quarterly average) of its assets is attributable to assets that produce or are held for the production of passive income. |
For this purpose, passive income generally includes dividends, interest, royalties and rents (other than royalties and rents derived in the active conduct of a trade or business and not derived from a related person). If the Corporation owns at least 25% (by value) of the stock of another corporation, it will be treated, for purposes of the PFIC tests, as owning its proportionate share of the other corporation’s assets and receiving its proportionate share of the other corporation’s income.
If the Corporation is a PFIC for any taxable year during which a U.S. Holder holds the Common Shares, the U.S. Holder will be subject to special tax rules with respect to any “excess distribution” received and any gain realized from a sale or other disposition, including a pledge, of the Common Shares. Distributions received in a taxable year that are greater than 125% of the average annual distributions received during the shorter of the three preceding taxable years or a U.S. Holder’s holding period for the Common Shares will be treated as excess distributions. Under these special tax rules:
| · | the excess distribution or gain will be allocated ratably over a U.S. Holder’s holding period for the Common Shares, |
| · | the amount allocated to the current taxable year, and any taxable year prior to the first taxable year in which the Corporation was a PFIC, will be treated as ordinary income, and |
| · | the amount allocated to each other year will be subject to tax at the highest ordinary income tax rate in effect for that year and the interest charge generally applicable to underpayments of tax will be imposed on the resulting tax attributable to each such year. |
S-46
If the Corporation is a PFIC for any year during which a U.S. Holder holds Common Shares, the Corporation will generally continue to be treated as a PFIC with respect to such holder for all subsequent years during which such Common Shares continue to be held, even if the Corporation ceases to meet the threshold requirements for PFIC status. U.S. Holders should consult with their own tax advisors regarding the availability of a “deemed sale” election that in certain circumstances would allow such holder to terminate PFIC status with respect to such Common Shares.
If the Corporation is a PFIC for any taxable year during which a U.S. Holder holds Common Shares and any of the Corporation’s non-U.S. subsidiaries is also a PFIC, or a lower-tier PFIC, a U.S. Holder would be treated as owning a proportionate amount (by value) of the shares of the lower-tier PFIC for purposes of the application of these rules on (i) excess distributions by the lower-tier PFIC, and (ii) a disposition of shares of a lower-tier PFIC, in each case as if the U.S. Holder held such shares directly, even though the holders have not received the proceeds of those distributions or dispositions directly.
In lieu of being subject to the excess distribution rules discussed above with respect to the Common Shares (but not with respect to any lower-tier PFIC), a U.S. Holder may make an election to use the mark-to-market method, provided that such stock is regularly traded on a qualified exchange. The Common Shares will be treated as “regularly traded” in any calendar year in which more than a de minimis quantity of such shares are traded on a qualified exchange on at least 15 days during each calendar quarter of such year. The Nasdaq on which the Common Shares are expected to be traded is a qualified exchange for this purpose.
If a U.S. Holder makes an effective mark-to-market election, it will include in each year the Corporation is a PFIC as ordinary income the excess of the fair market value of such U.S. Holder’s Common Shares at the end of the year over the U.S. Holder’s adjusted tax basis in the Common Shares. A U.S. Holder will be entitled to deduct as an ordinary loss in each such year the excess of the U.S. Holder’s adjusted tax basis in the Common Shares over their fair market value at the end of the year, but only to the extent of the net amount previously included in income as a result of the mark-to-market election. If a U.S. Holder makes an effective mark-to-market election, any gain the U.S. Holder recognizes upon the sale or other disposition of its Common Shares in a year that the Corporation is a PFIC it will be treated as ordinary income and any loss will be treated as ordinary loss, but only to the extent of the net amount of previously included income as a result of the mark-to-market election, and any additional loss will be treated as a capital loss.
A U.S. Holder’s adjusted tax basis in its Common Shares will be increased by the amount of any income inclusion and decreased by the amount of any deductions under the mark-to-market rules. If a U.S. Holder makes a mark-to-market election, it will be effective for the taxable year for which the election is made and all subsequent taxable years that the Corporation is a PFIC unless the Common Shares are no longer regularly traded on a qualified exchange or the IRS consents to the revocation of the election. A mark-to-market election will not apply to our Common Shares for any taxable year in which the Corporation is not a PFIC, but will remain in effect with respect to any subsequent taxable year in which the Corporation becomes a PFIC. U.S. Holders are urged to consult their tax advisors about the availability of the mark-to-market election, and whether making the election would be advisable in their particular circumstances.
Alternatively, a U.S. Holder may avoid the rules described above by electing to treat the Corporation (and any lower-tier PFIC) as a “qualified electing fund,” or QEF, under Section 1295 of the Code. A QEF election requires a U.S. Holder to include currently in income each year its pro rata share of a PFIC’s ordinary earnings and net capital gains, regardless of whether or not such ordinary earnings and gains are actually distributed. Thus, a U.S. Holder could have a tax liability with respect to such ordinary earnings or gains without a corresponding receipt of cash. A U.S. Holder’s basis in the shares of a QEF will be increased to reflect the amount of the taxed but undistributed income. Distributions of income that had previously been taxed will result in a corresponding reduction of basis in the shares and will not be taxed again as a distribution to the U.S. Holder. In addition, a U.S. Holder will recognize capital gain or loss on the disposition of Common Shares in an amount equal to the difference between the amount realized and the holder’s adjusted tax basis in the Common Shares. To make a QEF election, a U.S. Holder will need to have an annual information statement from the PFIC setting forth the earnings and capital gains for the year. U.S. Holders should consult their own tax advisors as to the consequences of making a protective QEF election or other consequences of the QEF election. Upon request of a U.S. Holder, the Corporation will provide the information necessary for a U.S. Holder to make the QEF election. However, no assurance can be given that such QEF information will be available for any lower-tier PFIC that the Corporation does not control.
S-47
U.S. Holders will be required to file IRS Form 8621 if they hold the Common Shares in any year in which the Corporation is classified as a PFIC.
U.S. Holders are urged to consult their tax advisors concerning the United States federal income tax consequences of holding the Common Shares if the Corporation or its subsidiaries are considered a PFIC in any taxable year and concerning the consequences of making a protective QEF election or other consequences of the QEF election or any other United States federal income tax election described herein.
Additional Considerations
Additional Tax on Passive Income
Individuals, estates and certain trusts whose income exceeds certain thresholds will be required to pay a 3.8% Medicare surtax on “net investment income” including, among other things, dividends and net gain from disposition of property (other than property held in certain trades or businesses). Special rules apply to PFICs. U.S. Holders should consult with their own tax advisors regarding the effect, if any, of this tax on their ownership and disposition of Common Shares.
Receipt of Foreign Currency
The amount of any distribution paid in Canadian dollars to a U.S. Holder in connection with the ownership of the Common Shares, or on the sale, exchange or other taxable disposition of Common Shares, will be included in the gross income of a U.S. Holder as translated into U.S. dollars calculated by reference to the exchange rate prevailing on the date of actual or constructive receipt of the payment, regardless of whether the Canadian dollars are converted into U.S. dollars at that time. If the Canadian dollars received are not converted into U.S. dollars on the date of receipt, a U.S. Holder will have a basis in the Canadian dollars equal to their U.S. dollar value on the date of receipt. Any U.S. Holder who receives payment in Canadian dollars and engages in a subsequent conversion or other disposition of the Canadian dollars may have a foreign currency exchange gain or loss that would be treated as ordinary income or loss, and generally will be U.S. source income or loss for foreign tax credit purposes. Different rules apply to U.S. Holders who use the accrual method with respect to foreign currency. Each U.S. Holder should consult its own U.S. tax advisor regarding the U.S. federal income tax consequences of receiving, owning, and disposing of Canadian dollars.
Foreign Tax Credit
Subject to the PFIC rules discussed above, a U.S. Holder that pays (whether directly or through withholding) Canadian income tax with respect to dividends paid on the Common Shares generally will be entitled, at the election of such U.S. Holder, to receive either a deduction or a credit for such Canadian income tax paid. Generally, a credit will reduce a U.S. Holder’s U.S. federal income tax liability on a dollar-for-dollar basis, whereas a deduction will reduce a U.S. Holder’s income subject to U.S. federal income tax. This election is made on a year-by-year basis and applies to all foreign taxes paid (whether directly or through withholding) by a U.S. Holder during a year. Complex limitations apply to the foreign tax credit, including the general limitation that the credit cannot exceed the proportionate share of a U.S. Holder’s U.S. federal income tax liability that such U.S. Holder’s “foreign source” taxable income bears to such U.S. Holder’s worldwide taxable income. In applying this limitation, a U.S. Holder’s various items of income and deduction must be classified, under complex rules, as either “foreign source” or “U.S. source”. Generally, dividends paid by a foreign corporation should be treated as foreign source for this purpose, and gains recognized on the sale of stock of a foreign corporation by a U.S. Holder should be treated as U.S. source for this purpose, except as otherwise provided in an applicable income tax treaty, and if an election is properly made under the Code. However, the amount of a distribution with respect to the Common Shares that is treated as a “dividend” may be lower for U.S. federal income tax purposes than it is for Canadian federal income tax purposes, resulting in a reduced foreign tax credit allowance to a U.S. Holder. In addition, this limitation is calculated separately with respect to specific categories of income. The foreign tax credit rules are complex, and each U.S. Holder should consult its own U.S. tax advisor regarding the foreign tax credit rules.
Special rules apply to the amount of foreign tax credit that a U.S. Holder may claim on a distribution from a PFIC. Subject to such special rules, non-U.S. taxes paid with respect to any distribution in respect of stock in a PFIC are generally eligible for the foreign tax credit. The rules relating to distributions by a PFIC and their eligibility for the foreign tax credit are complex, and a U.S. Holder should consult its own tax advisor regarding their application to the U.S. Holder.
S-48
Backup Withholding and Information Reporting
Under U.S. federal income tax law and Treasury Regulations, certain categories of U.S. Holders must file information returns with respect to their investment in, or involvement in, a non-U.S. corporation. For example, U.S. return disclosure obligations (and related penalties) are imposed on U.S. Holders that hold certain specified foreign financial assets in excess of certain threshold amounts. The definition of “specified foreign financial assets” includes not only financial accounts maintained in foreign financial institutions, but also, if held for investment and not in an account maintained by certain financial institutions, any stock or security issued by a non-U.S. person, any financial instrument or contract held for investment that has an issuer or counterparty other than a U.S. person and any interest in a foreign entity. U.S. Holders may be subject to these reporting requirements unless their Common Shares are held in an account at certain financial institutions. Penalties for failure to file certain of these information returns are substantial. U.S. Holders should consult with their own tax advisors regarding the requirements of filing information returns on IRS Form 8938.
Payments made within the U.S. or by a U.S. payor or U.S. middleman of (a) distributions on the Common Shares, and (b) proceeds arising from the sale or other taxable disposition of Common Shares generally will be subject to information reporting. In addition, backup withholding, currently at a rate of 24% may apply to such payments if a U.S. Holder (a) fails to furnish such U.S. Holder’s correct U.S. taxpayer identification number (generally on IRS Form W-9), (b) furnishes an incorrect U.S. taxpayer identification number, (c) is notified by the IRS that such U.S. Holder has previously failed to properly report items subject to backup withholding, or (d) fails to certify, under penalty of perjury, that such U.S. Holder has furnished its correct U.S. taxpayer identification number and that the IRS has not notified such U.S. Holder that it is subject to backup withholding. Certain exempt persons generally are excluded from these information reporting and backup withholding rules. Backup withholding is not an additional tax. Any amounts withheld under the U.S. backup withholding rules will be allowed as a credit against a U.S. Holder’s U.S. federal income tax liability, if any, or will be refunded, if such U.S. Holder furnishes required information to the IRS in a timely manner. The information reporting and backup withholding rules may apply even if, under the Canada-U.S. Tax Convention, payments are exempt from the dividend withholding tax or otherwise eligible for a reduced withholding rate. This discussion of reporting requirements set forth above is not intended to constitute an exhaustive description of all reporting requirements that may apply to a U.S. Holder. A failure to satisfy certain reporting requirements may result in an extension of the time period during which the IRS can assess a tax, and under certain circumstances, such an extension may apply to assessments of amounts unrelated to any unsatisfied reporting requirements. Each U.S. Holder should consult its own tax advisor regarding the information reporting and backup withholding rules.
THE ABOVE SUMMARY IS NOT INTENDED TO CONSTITUTE A COMPLETE ANALYSIS OF ALL U.S. TAX CONSIDERATIONS APPLICABLE TO U.S. HOLDERS WITH RESPECT TO THE OWNERSHIP AND DISPOSITION OF COMMON SHARES. U.S. HOLDERS SHOULD CONSULT THEIR OWN TAX ADVISORS AS TO THE TAX CONSIDERATIONS APPLICABLE TO THEM IN THEIR PARTICULAR CIRCUMSTANCES.
Certain Canadian legal matters relating to the Offering will be passed upon on behalf of the Corporation by McCarthy Tétrault LLP and on behalf of Piper by Blake, Cassels & Graydon LLP. As of the date hereof, the partners and associates of McCarthy Tétrault LLP, as a group, and the partners and associates of Blake, Cassels & Graydon LLP, as a group, beneficially own, directly or indirectly, less than 1% of the outstanding Common Shares.
Certain legal matters relating to United States law will be passed upon on behalf of the Corporation by Troutman Sanders LLP and on behalf of Piper Sandler by Cooley LLP.
S-49
AUDITOR, TRANSFER AGENT AND REGISTRAR
The auditor of the Corporation is PricewaterhouseCoopers LLP, Chartered Professional Accountants, Halifax, Nova Scotia, Canada. PricewaterhouseCoopers LLP has confirmed that they are independent with respect to the Corporation within the meaning of the relevant rules and related interpretations prescribed by the relevant professional bodies in Canada and any applicable legislation or regulation and within the meaning of the U.S. Securities and Exchange Commission.
The transfer agent and registrar for the Common Shares is Computershare Investor Services Inc., at its principal offices located in Toronto, Ontario, Canada or Montreal, Quebec, Canada.
Julia Gregory, Wayne Pisano and Markus Warmuth, directors of the Corporation, all reside outside of Canada and have appointed IMV Inc., 130 Eileen Stubbs Avenue, Suite 19, Dartmouth, Nova Scotia, Canada, B3B 2C4, as agent for service of process.
Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process.
STATUTORY RIGHTS OF WITHDRAWAL AND RESCISSION
Securities legislation in certain of the provinces of Canada provides purchasers with the right to withdraw from an agreement to purchase securities. This right may be exercised within two business days after receipt or deemed receipt of a prospectus and any amendment. In several of the provinces of Canada, the securities legislation further provides a purchaser with remedies for rescission or, in some jurisdictions, revisions of the price or damages if the prospectus and any amendment contains a misrepresentation or is not delivered to the purchaser, provided that the remedies for rescission, revision of the price or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province for the particulars of these rights or consult with a legal advisor. Rights and remedies may also be available to purchasers under U.S. law; purchasers may wish to consult with a U.S. lawyer for particulars of these rights.
The Corporation is incorporated under, and governed by, the laws of Canada. Many of its officers and directors and experts named in this Prospectus Supplement and the Base Shelf Prospectus are resident outside of the United States, and a majority of their assets, and the assets of IMV, are located outside the United States. As a result, it may be difficult for U.S. investors to effect service of process within the United States upon those directors, officers or experts who are not residents of the United States, or to realize in the United States upon judgments of courts of the United States predicated upon civil liability of such directors, officers or experts under U.S. federal securities laws. There is doubt as to whether Canadian courts would enforce the civil liability claims brought under United States federal securities laws in original actions and/or enforce claims for punitive damages. A final judgment for a liquidated sum in favour of a private litigant granted by a United States court and predicated solely upon civil liability under United States federal securities laws would, subject to certain exceptions identified in the law of individual provinces of Canada, likely be enforceable in Canada if the United States court in which the judgment was obtained had a basis for jurisdiction in the matter that would be recognized by the domestic Canadian court for the same purposes. There is a significant risk that a given Canadian court may not have jurisdiction or may decline jurisdiction over a claim based solely upon United States federal securities law on application of the conflict of laws principles of the province in Canada in which the claim is brought.
IMV has filed with the SEC, concurrently with the filing of its U.S. Registration Statement on Form F-10 of which this Prospectus Supplement and the Base Shelf Prospectus form a part, an appointment of agent for service of process on Form F-X. Under the Form F-X, IMV appointed C T Corporation System as its agent for service of process in the United States in connection with any investigation or administrative proceeding conducted by the SEC, and any civil suit or action brought against or involving IMV in a U.S. court arising out of or related to or concerning the Offering of Offered Shares under the U.S. Registration Statement. However, it may be difficult for United States investors to effect service of process within the United States upon those officers or directors who are not residents of the United States, or to realize in the United States upon judgments of courts of the United States predicated upon the Corporation’s civil liability and the civil liability of such officers or directors under United States federal securities laws or the securities or “blue sky” laws of any state within the United States.
S-50
Base Shelf Prospectus
This short form base shelf prospectus has been filed under legislation in the provinces British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Québec, Nova Scotia and Newfoundland and Labrador that permits certain information about these securities to be determined after this prospectus has become final and that permits the omission from this prospectus of that information. The legislation requires the delivery to purchasers of a prospectus supplement containing the omitted information within a specified period of time after agreeing to purchase any of these securities.
Information contained herein is subject to completion or amendment. A registration statement relating to these securities has been filed with the United States Securities and Exchange Commission. These securities may not be offered or sold nor may offers to buy be accepted prior to the time the registration statement becomes effective. This short form prospectus shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of these securities in any state in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state.
No securities regulatory authority has expressed an opinion about these securities and it is an offence to claim otherwise. This short form base shelf prospectus constitutes a public offering of these securities only in those jurisdictions where they may be lawfully offered for sale and therein only by persons permitted to sell such securities.
Information has been incorporated by reference in this short form prospectus from documents filed with securities commissions or similar authorities in Canada. Copies of the documents incorporated herein by reference may be obtained on request without charge from the Corporate Secretary of IMV Inc. at #53-1344 Summer Street, Suite 412, Halifax, Nova Scotia, Canada, B3H 0A8 (telephone (902) 492-1819), and are also available electronically at www.sedar.com.
SHORT FORM BASE SHELF PROSPECTUS
| New Issue | June 5, 2018 |

$150,000,000
Preferred Shares
Common Shares
Subscription Receipts
Warrants
Units
Under this short form base shelf prospectus (the “Prospectus”), IMV Inc. (formerly Immunovaccine Inc.) (“IMV” or the “Corporation”) may, from time to time during the 25-month period that this Prospectus, including any amendments, remains valid, offer and issue preferred shares (the “Preferred Shares”) or common shares (the “Common Shares”) of its share capital, or subscription receipts (the “Subscription Receipts”), warrants or options to purchase Common Shares (collectively, the “Warrants”) or units comprised of one or more of the other securities described in this Prospectus in any combination (the “Units” and together with the Common Shares, Subscription Receipts and Warrants, the “Securities”) in one or more offerings of up to $150,000,000 (or the equivalent in foreign currencies). The Securities may be offered separately or together, in amounts, at prices and on terms based on market conditions at the time of the sale and set forth in an accompanying prospectus supplement (a “Prospectus Supplement”). The Corporation may sell the Preferred Shares, the Subscription Receipts and the Warrants in one or more series.
The specific variable terms of any offering of Securities will be set forth in a Prospectus Supplement and may include, where applicable:
| - | in the case of Preferred Shares, the number of Preferred Shares offered, the offering price and any other specific terms; |
| - | in the case of Common Shares, the number of Common Shares offered, the offering price and any other specific terms; |
| - | in the case of Subscription Receipts, the number of Subscription Receipts offered, the issue price, the terms and procedures for the exchange of the Subscription Receipts and any other specific terms; |
| - | in the case of Warrants, the number of Warrants offered, the offering price, the designation, number and terms of the Securities that may be purchased upon exercise of each Warrant and any other specific terms; and |
| - | in the case of Units, the designation, number and terms of any other Securities comprising, in any combination, the Units. |
A Prospectus Supplement may include specific variable terms pertaining to the Securities that are not within the alternatives and parameters set forth in this Prospectus.
All shelf information permitted under Securities legislation to be omitted from this Prospectus will be contained in one or more Prospectus Supplements that will be delivered to purchasers together with this Prospectus. Each Prospectus Supplement will be incorporated by reference into this Prospectus for the purposes of Securities legislation as of the date of the Prospectus Supplement and only for the purposes of the distribution of the Securities to which the Prospectus Supplement pertains. This Prospectus and any applicable Prospectus Supplement should be read carefully before investing in Securities. This Prospectus may not be used to offer any of the Securities unless accompanied by a Prospectus Supplement.
The Common Shares are listed on the Toronto Stock Exchange (the “TSX”) under the symbol “IMV” and on the Nasdaq Capital Market (“NASDAQ”) under the symbol “IMV”. On June 4, 2018, the last trading day of the Common Shares on the TSX and the NASDAQ before the date hereof, the closing price of the Common Shares was $8.26 and US$6.879, respectively. Unless otherwise specified in an applicable Prospectus Supplement, the Preferred Shares, the Subscription Receipts, the Warrants and the Units will not be listed on any securities or stock exchange or on any automated dealer quotation system.
The Corporation may offer and sell Securities to or through underwriters, dealers, placement agents or other intermediaries and the Corporation may also offer and sell its Securities directly to one or more purchasers, or through agents designated from time to time at amounts and prices and other terms determined by the Corporation. The Prospectus Supplement relating to a particular offering of Securities will identify each underwriter, dealer, placement agent, intermediary or agent engaged in connection with the offering and sale of Securities and will set forth the plan of distribution for such Securities, including the proceeds to the Corporation and any fees, discounts, concessions or other compensation payable to the underwriters, dealers or agents, and any other material terms of the plan of distribution. See “Plan of Distribution”.
The offering of Securities hereunder is made by a Canadian issuer that is permitted, under a multijurisdictional disclosure system (“MJDS”) adopted by the United States and Canada, to prepare this Prospectus in accordance with Canadian disclosure requirements. Prospective investors should be aware that such requirements are different from those of the United States. Annual financial statements for the year ended December 31, 2017 included or incorporated herein have been prepared in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board (“IFRS”) and are subject to Canadian auditing and auditor independence standards and thus may not be comparable to financial statements of United States companies.
The enforcement by investors of civil liabilities under the United States federal Securities laws may be affected adversely by the fact that the Corporation is incorporated or organized under the laws of a foreign country, that some or all of its officers and directors may be residents of a foreign country, that some or all of the underwriters or experts named in this Prospectus or any Prospectus Supplement may by residents of a foreign country and that all or a substantial portion of the assets of the Corporation and said persons may be located outside the United States.
| ii |
THESE SECURITIES HAVE NOT BEEN APPROVED OR DISAPPROVED BY THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION (“SEC”) NOR HAS THE SECURITIES COMMISSION OF ANY STATE OF THE UNITED STATES OR ANY CANADIAN SECURITIES REGULATOR APPROVED OR DISAPPROVED THESE SECURITIES OR PASSED UPON THE ACCURACY OR ADEQUACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
Investors should be aware that the acquisition, holding or disposition of the Securities described herein may have tax consequences both in the United States and in Canada. Such consequences for investors who are resident in, or citizens of, the United States and Canada may not be described fully herein. You should read the tax discussion contained in the applicable Prospectus Supplement with respect to a particular offering of Securities and consult your own tax advisor with respect to your own particular circumstances.
Albert Scardino and Wayne Pisano, members of the board of directors of the Corporation, both reside outside of Canada and have appointed IMV Inc., #53-1344 Summer Street, Suite 412, Halifax, Nova Scotia, Canada, BH3 0A8, as agent for service of process. Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process.
The Corporation’s head office and registered office is located at #53-1344 Summer Street, Suite 412, Halifax, Nova Scotia, Canada, BH3 0A8.
Investing in the Securities involves risks, including those that are described in the “Risk Factors” section of this Prospectus. The Corporation will apply to list the Common Shares distributed under this Prospectus including the Common Shares underlying the Preferred Shares, Units, Warrants and Subscription Receipts, if any. However, unless specified in the applicable Prospectus Supplement, there is no market through which the Preferred Shares, Units, Warrants and Subscriptions Receipts may be sold and purchasers may not be able to resell the Preferred Shares, Units, Warrants and Subscription Receipts purchased under this Prospectus and the Prospectus Supplements. This may affect the pricing of the Preferred Shares, Units, Warrants and Subscription Receipts in the secondary market, the transparency and availability of trading prices, the liquidity of the Preferred Shares, Units, Warrants and Subscription Receipts and the extent of issuer regulation. See “Risk Factors”.
No underwriter, dealer, placement agent, other intermediary or agent has been involved in the preparation of this Prospectus or performed any review of the contents of this Prospectus.
On May 2, 2018, the Corporation filed articles of amendment to give effect to a consolidation of its Common Shares on the basis of 1 post-consolidation Common Share for each 3.2 pre-consolidation Common Shares. The post-consolidation Common Shares began trading on TSX on May 10, 2018. Historical trading prices and volumes have been amended to reflect the 3.2 for 1 consolidation.
The offering of Securities hereunder is subject to approval of certain legal matters on behalf of the Corporation by McCarthy Tétrault LLP, with respect to Canadian legal matters, and by Troutman Sanders LLP, with respect to U.S. legal matters.
| iii |
TABLE OF CONTENTS
Purchasers of Securities should rely only on the information contained in or incorporated by reference into this Prospectus or any applicable Prospectus Supplement. The Corporation has not authorized anyone to provide purchasers with different or additional information. If anyone provides purchasers with different or additional information, purchasers should not rely on it. The Corporation is not making an offer to sell or seeking an offer to buy these Securities in any jurisdiction where the offer or sale is not permitted. Purchasers should assume that the information contained in this Prospectus or any applicable Prospectus Supplement is accurate only as of the date on the front of those documents and that information contained in any document incorporated by reference is accurate only as of the date of that document, regardless of the time of delivery of this Prospectus or any applicable Prospectus Supplement or of any sale of the Securities. The Corporation’s business, financial condition, results of operations and prospects may have changed since those dates.
“DepoVax” is a trademark of the Corporation. This Prospectus also includes references to trade names and trademarks of other companies, which trade names and trademarks are the properties of their respective owners.
The corporate website of the Corporation is www.imv-inc.com. The information on the Corporation’s website is not intended to be included or incorporated by reference into this Prospectus and prospective purchasers should not rely on such information when deciding whether or not to invest in the Securities.
Statistical information and other data relating to the pharmaceutical and biotechnology industry included in this Prospectus are derived from recognized industry reports published by industry analysts, industry associations and/or independent consulting and data compilation organizations. Market data and industry forecasts used throughout this Prospectus were obtained from various publicly available sources. Although the Corporation believes that these independent sources are generally reliable, the accuracy and completeness of the information from such sources are not guaranteed and have not been independently verified.
In this Prospectus, unless otherwise noted, all dollar amounts are expressed in Canadian dollars.
This Prospectus is part of a registration statement on Form F-10 (the “U.S. Registration Statement”) relating to the Securities that the Corporation has or will file with the SEC. Under the U.S. Registration Statement, the Corporation may, from time to time, sell Securities described in this Prospectus in one or more offerings up to an aggregate offering amount of $150,000,000. This Prospectus, which constitutes part of the U.S. Registration Statement, provides you with a general description of the Securities that the Corporation may offer. Each time the Corporation sells Securities under the U.S. Registration Statement, it will provide a Prospectus Supplement that will contain specific information about the terms of that offering of Securities. A Prospectus Supplement may also add, update or change information contained in this Prospectus. Before you invest, you should read both this Prospectus and any applicable Prospectus Supplement together with additional information described under the heading “Documents Incorporated by Reference”. This Prospectus does not contain all of the information set forth in the U.S. Registration Statement, certain parts of which are omitted in accordance with the rules and regulations of the SEC, or the schedules or exhibits that are part of the U.S. Registration Statement. Investors in the United States should refer to the U.S. Registration Statement and the exhibits thereto for further information with respect to IMV and the Securities.
EXCHANGE RATE INFORMATION
The consolidated financial statements incorporated by reference into this Prospectus and the other documents incorporated by reference into this Prospectus, and the financial data derived from those consolidated financial statements included in this Prospectus, are presented in Canadian dollars, unless otherwise specified, and have been prepared in accordance with IFRS. References in this Prospectus to “dollars”, “Cdn$” or “$” are to Canadian dollars. United States dollars are indicated by the symbol “US$”.
The following table lists, for each period presented, the high and low exchange rates, the average of the exchange rates during the period indicated, and the exchange rates at the end of the period indicated, for one Canadian dollar, expressed in United States dollars, based on the closing exchange rate published by the Bank of Canada for the applicable periods.
| Year ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| High for the period | 0.8245 | 0.7977 | 0.8511 | |||||||||
| Low for the period | 0.7276 | 0.6869 | 0.7161 | |||||||||
| End of period | 0.7971 | 0.7448 | 0.7821 | |||||||||
| Average for the period | 0.7701 | 0.7550 | 0.7225 | |||||||||
On June 4, 2018, the closing exchange rate for one Canadian dollar, expressed in United States dollars, as reported by the Bank of Canada, was Cdn$1.00 = US$0.7735.
Cautionary Statement regarding Forward-Looking Statements
Certain statements contained in this Prospectus, any Prospectus Supplement and the documents incorporated by reference herein and therein may constitute “forward-looking information” within the meaning of applicable securities laws in Canada and “forward-looking statements” within the meaning of the United States Private Securities Litigation Reform Act of 1995 (collectively, “forward-looking statements”) which involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements of the Corporation, or industry results, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. When used in this Prospectus, such statements reflect current expectations regarding future events and operating performance and speak only as of the date of this Prospectus. Forward-looking statements may use such words as “will”, “may”, “could”, “intends”, “potential”, “plans”, “believes”, “expects”, “projects”, “estimates”, “anticipates”, “continue”, “potential”, “predicts” or “should” and other similar terminology.
Forward-looking statements include, but are not limited to, statements relating to:
| - | the Corporation’s business strategy; |
| - | statements with respect to the sufficiency of the Corporation’s financial resources to support its activities; |
| - | potential sources of funding; |
| - | the Corporation’s ability to obtain necessary funding on favorable terms or at all; |
| - | the Corporation’s expected expenditures and accumulated deficit level; |
| - | the Corporation’s expected outcomes from its ongoing and future research and research collaborations; |
| - | the Corporation’s exploration of opportunities to maximize shareholder value as part of the ordinary course of its business through collaborations, strategic partnerships and other transactions with third parties; |
| - | the Corporation’s plans for the research and development of certain product candidates; |
| - | the Corporation’s strategy for protecting its intellectual property; |
| - | the Corporation’s ability to identify licensable products or research suitable for licensing and commercialization; |
| - | the Corporation’s ability to obtain licences on commercially reasonable terms; |
| - | the Corporation’s plans for generating revenue; |
| - | the Corporation’s plans for future clinical trials; and |
| - | the Corporation’s hiring and retention of skilled staff. |
The forward-looking statements reflect the Corporation’s current views with respect to future events, are subject to risks and uncertainties, and are based upon a number of estimates and assumptions that, while considered reasonable by the Corporation, are inherently subject to significant business, economic, competitive, political and social uncertainties and contingencies. Many factors could cause the Corporation’s actual results, performance or achievements to be materially different from any future results, performance, or achievements that may be expressed or implied by such forward-looking statements, including, among others:
| 2 |
| - | obtaining additional funding on reasonable terms when necessary; |
| - | positive results of pre-clinical and clinical tests; |
| - | the Corporation’s ability to successfully develop existing and new products; |
| - | the Corporation’s ability to hire and retain skilled staff; |
| - | the products and technology offered by the Corporation’s competitors; |
| - | general business and economic conditions; |
| - | the Corporation’s ability to protect its intellectual property; |
| - | the Corporation’s ability to manufacture its products and to meet demand; and |
| - | regulatory approvals. |
Should one or more of these risks or uncertainties materialize, or should the assumptions set out in the section entitled “Risk Factors” underlying those forward-looking statements prove incorrect, actual results may vary materially from those described herein. These forward-looking statements are made as of the date of this Prospectus or, in the case of documents incorporated by reference in this Prospectus, as of the date of such documents, and the Corporation does not intend, and does not assume any obligation, to update these forward-looking statements, except as required by law. There is no assurance that such statements will prove to be accurate as actual results and future events could differ materially from those anticipated in such statements. Purchasers are cautioned that forward-looking statements are not guarantees of future performance and accordingly purchasers are cautioned not to put undue reliance on forward-looking statements due to the inherent uncertainty therein. New factors emerge from time to time, and it is not possible for management of the Corporation to predict all of these factors or to assess in advance the impact of each such factor on the Corporation’s business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statement.
The forward-looking statements contained in this Prospectus are expressly qualified by the foregoing cautionary statements and are made as of the date of this Prospectus. The Corporation does not undertake any obligation to publicly update or revise any forward-looking statements, except as required by applicable securities laws. Purchasers should read this Prospectus and consult their own professional advisors to assess the income tax, legal, risk factors and other aspects of their investment in the securities.
DOCUMENTS INCORPORATED BY REFERENCE
Information has been incorporated by reference in this Prospectus from documents filed with securities commissions or similar authorities in Canada. Copies of the documents incorporated herein by reference may be obtained on request without charge from the Corporate Secretary of the Corporation at #53-1344 Summer Street, Suite 412, Halifax, Nova Scotia, Canada, B3H 0A8 (telephone (902) 492-1819), and are also available electronically at www.sedar.com.
In addition to the continuous disclosure obligations of the Corporation under the securities laws of certain provinces of Canada, the Corporation is subject to certain of the information requirements of the U.S. Securities Exchange Act of 1934, as amended (the “Exchange Act”), and in accordance therewith file reports and other information with the SEC. Under MJDS, some reports and other information may be prepared in accordance with the disclosure requirements of Canada, which requirements are different from those of the United States. As a foreign private issuer, the Corporation is exempt from the rules under the Exchange Act prescribing the furnishing and content of proxy statements, and the Corporation’s officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, the Corporation may not be required to publish financial statements as promptly as U.S. companies. You may read any document that the Corporation files with or furnish to the SEC at the SEC’s public reference room at Room 1580, 100 F Street N.E., Washington, D.C. 20549. You may also obtain copies of the same documents from the public reference room of the SEC at 100 F Street N.E., Washington D.C. 20549 by paying a fee. You should call the SEC at 1-800-SEC-0330 or access its website at www.sec.gov for further information about the public reference room. As well, a free copy of any public document filed by IMV with the SEC’s Electronic Data Gathering and Retrieval (EDGAR) system is available from the SEC’s website at www.sec.gov.
| 3 |
The following documents filed with the securities commissions or similar authorities in the provinces of British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Québec, Nova Scotia and Newfoundland and Labrador are specifically incorporated by reference in and form an integral part of this Prospectus:
| (i) | the annual information form of the Corporation dated March 20, 2018 for the year ended December 31, 2017 (the “AIF”); |
| (ii) | the audited annual consolidated financial statements of the Corporation and the notes thereto for the years ended December 31, 2017 and 2016, together with the auditor’s report thereon; |
| (iv) | the unaudited interim condensed consolidated financial statements of the Corporation and the notes thereto for the three months ended March 31, 2018 and 2017; |
| (vi) | the management information circular dated March 29, 2018 relating to the annual and special meeting of shareholders of the Corporation held on May 1, 2018; |
| (vii) | the material change report dated February 2, 2018 relating to a bought-deal financing agreement to sell Common Shares (the “February 2018 Public Offering”); |
| (viii) | the material change report dated February 21, 2018 relating to the closing of the February 2018 Public Offering; and |
| (ix) | the material change report dated May 10, 2018 relating the NASDAQ listing, the consolidation of the Common Shares and the name change. |
Any documents of the Corporation of the type referred to in the preceding paragraph and any material change reports (excluding any confidential material change reports) filed by the Corporation with a securities commission or similar regulatory authority in Canada on or after the date of Prospectus and prior to the termination of the offering of Securities hereunder shall be deemed to be incorporated by reference into this Prospectus.
In addition, to the extent that any document or information incorporated by reference into this Prospectus is included in any report on Form 6-K, Form 40-F or Form 20-F (or any respective successor form) that is filed with or furnished to the SEC by the Corporation after the date of this Prospectus, such document or information shall be deemed to be incorporated by reference as an exhibit to the U.S. Registration Statement of which this Prospectus forms a part. In addition, the Corporation may incorporate by reference into this Prospectus, or the U.S. Registration Statement of which it forms a part, other information from documents that the Corporation will file with or furnish to the SEC pursuant to Section 13(a) or 15(d) of the Exchange Act, if and to the extent expressly provided therein.
Any statement contained in this Prospectus or in a document incorporated or deemed to be incorporated by reference in this Prospectus shall be deemed to be modified or superseded for purposes of this Prospectus to the extent that a statement contained herein or in any other subsequently filed document which also is or is deemed to be incorporated by reference in this Prospectus modifies or supersedes such statement. The modifying or superseding statement need not state that it has modified or superseded a prior statement or include any other information set forth in the document that it modifies or supersedes. Any statement so modified or superseded shall not be deemed to constitute a part of this Prospectus, except as so modified or superseded.
You should rely only on the information contained in or incorporated by reference in this Prospectus or any applicable Prospectus Supplement and on the other information included in the U.S. Registration Statement of which this Prospectus forms a part. The Corporation is not making an offer of Securities in any jurisdiction where the offer is not permitted by law.
| 4 |
DOCUMENTS FILED AS PART OF THE U.S. REGISTRATION STATEMENT
The following documents have been filed with the SEC as part of the U.S. Registration Statement of which this Prospectus is a part insofar as required by the SEC’s Form F-10:
| · | the documents listed under “Documents Incorporated by Reference” in this Prospectus; |
| · | the consent of PricewaterhouseCoopers LLP, the Corporation’s independent auditor; |
| · | the consent of McCarthy Tétrault LLP, the Corporation’s Canadian counsel; and |
| · | powers of attorney of the Corporation’s directors and officers, as applicable. |
A copy of the form of warrant indenture will be filed by post-effective amendment or by incorporation by reference to documents filed with or furnished to the SEC under the Exchange Act.
The Corporation was incorporated on May 18, 2007 under the name of Rhino Resources Inc. pursuant to the Canada Business Corporations Act. On September 28, 2009, the Corporation changed its name to Immunovaccine Inc. and consolidated its outstanding share capital on a 5 to 1 basis. On May 2, 2018, the Corporation changed its name to IMV Inc. and consolidated its outstanding share capital on a 3.2 to 1 basis.
The Corporation has one wholly-owned subsidiary, Immunovaccine Technologies Inc. (“IVT”), which is incorporated under the laws of Nova Scotia.
The Corporation’s head and registered office is located at 1344 Summer Street, Suite 412, Halifax, Nova Scotia, Canada, B3H 0A8.
Overview
IMV is a clinical-stage company pioneering a new class of immunotherapies based on a disruptive drug delivery technology (DPX) with potential applications in multiple markets in cancer, infectious diseases and other therapeutic areas. The DPX platform is based on a novel mechanism of action (MOA) for targeted delivery of active ingredients to immune cells using a patented lipid nanoparticle technology. The Corporation leverages this MOA to generate a new generation of therapeutic capabilities with a primary focus on T cell therapies for cancer.
The Corporation’s first cancer immunotherapy uses survivin-based peptides licensed from Merck KGaA, on a world-wide exclusive basis, formulated in DPX. Survivin is a well characterized and recognized tumor associated antigen known to be expressed during fetal development and across most tumour cell types, but is rarely present in normal, non-malignant adult cells. It has been shown that survivin was expressed in all 60 different human tumour lines used in the National Cancer Institute's cancer drug-screening program.
DPX-Survivac, is currently being tested in a co-funded Phase 1b clinical trial with Incyte Corporation (“Incyte”), which evaluates the combination of DPX-Survivac with Incyte’s investigational oral indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor, epacadostat, in ovarian cancer patients. DPX-Survivac is also being tested in two investigator-sponsored Phase 2 clinical trials in combination with checkpoint inhibitor pembrolizumab of Merck & Co Inc. in patients with recurrent, platinum-resistant and sensitive ovarian cancer and in patients with measurable or recurrent diffuse large B cell lymphoma (DLBCL). In infectious disease vaccine applications, the Corporation has completed a demonstration Phase 1 clinical trial with a target against the respiratory syncytial virus (RSV). The Corporation also has a commercial licencing agreement with Zoetis for the development of two cattle vaccines and is also conducting several research and clinical collaborations, including a collaboration with the Dana-Farber Cancer Institute for Human Papillomavirus (HPV) related cancers and with Leidos, Inc. in the United States for the development of vaccine candidates for malaria and the Zika virus.
| 5 |
Recent developments
On May 2, 2018, the Corporation filed articles of amendment to give effect to a consolidation of its Common Shares on the basis of 1 post-consolidation Common Share for each 3.2 pre-consolidation Common Shares. The post-consolidation Common Shares began trading on TSX on May 10, 2018.
The following table sets out certain information contained in documents incorporated by reference in this Prospectus for the periods indicated, restated as if the consolidation had occurred at such time. Due to the 3.2 for 1 share consolidation which occurred in May 2018, the total number of Common Shares and options outstanding, the total number of options exercisable and the weighted average Common Shares outstanding (basic and diluted) have all been divided by 3.2 from the numbers shown in the Corporation’s previously filed annual financial statements (reported on an annual basis). As a result of the consolidation, the basic and diluted earnings per Common Share have been multiplied by 3.2 from the numbers disclosed in the Corporation’s previously filed annual financial statements.
| December 31, 2017 | December 31, 2016 | |||||||
| Basic and diluted loss per share | (0.31 | ) | (0.28 | ) | ||||
| Common shares outstanding | 40,319,928 | 36,817,314 | ||||||
| Stock options outstanding | 1,498,044 | 1,961,765 | ||||||
| Warrants outstanding | 2,087,598 | 2,725,596 | ||||||
| Deferred share units outstanding | 186,327 | 101,563 | ||||||
| Weighted-average shares outstanding | 38,656,778 | 31,602,737 | ||||||
| Weighted-average shares outstanding - diluted | 38,656,778 | 31,602,737 | ||||||
On May 31, 2018, the Corporation announced that its Common Shares had been approved for listing on Nasdaq. Trading of the Common Shares on Nasdaq commenced on June 1, 2018 and concurrently the Common Shares ceased to be traded on the OTCQX.
On June 3, 2018, the Corporation announced that investigators shared new positive data in an oral presentation for its DeCidE1 (DPX-Survivac with low dose CyclophosphamIDe and Epacadostat) clinical study at the 2018 American Society for Clinical Oncology (ASCO) annual meeting. These data from the ongoing Phase 1b/2 trial evaluated the safety and efficacy of the combination of IMV’s lead candidate DPX-Survivac and low dose cyclophosphamide, with Incyte Corporation’s IDO1 enzyme inhibitor epacadostat, in patients with advanced recurrent ovarian cancer.
Except as otherwise disclosed in this Prospectus and the documents incorporated by reference herein, there have been no material changes in the consolidated share and loan capital of IMV from March 31, 2018 to the date of this Prospectus.
| 6 |
The aggregate proceeds of distributions of Securities under this Prospectus shall not exceed $150,000,000. The net proceeds to be received by the Corporation from the distribution from time to time of Securities under this Prospectus will be the gross proceeds of such issue less any commissions and expenses paid in connection therewith.
Unless otherwise specified in a Prospectus Supplement, the net proceeds received by the Corporation from the sale of the Securities will be used for working capital and general corporate purposes including, but not limited to, to advance the research and development and clinical advancement of the Corporation’s cancer and infectious disease vaccine candidates. A Prospectus Supplement will contain specific information about the use of proceeds from the sale of the Securities under that Prospectus Supplement.
More detailed information regarding the use of proceeds from the sale of the Securities will be described in any applicable Prospectus Supplement. Pending the application of the net proceeds, the Corporation intends to invest the net proceeds in investment-grade, interest-bearing securities, the primary objectives of which are liquidity and capital preservation.
Negative Cash Flow
The Corporation has incurred significant operating losses and negative cash flows from operations since inception and has an accumulated deficit of $70,820,867 as at December 31, 2017. The ability of the Corporation to continue as a going concern is dependent upon raising additional financing through equity and non-dilutive funding and partnerships. There can be no assurance that the Corporation will have sufficient capital to fund its ongoing operations, develop or commercialize any products without future financings. These material uncertainties cast significant doubt as to the Corporation’s ability to meet its obligations as they come due and, accordingly, the appropriateness of the use of accounting principles applicable to a going concern. If the Corporation is unable to obtain additional financing when required, the Corporation may have to substantially reduce or eliminate planned expenditures or the Corporation may be unable to continue operations.
The Corporation’s ability to continue as a going concern is dependent upon its ability to fund its research and development programs and defend its patent rights. It is expected that proceeds from the sale of Securities under the Prospectus will be used to fund anticipated negative cash flow from operating activities, as described above.
The Corporation may offer and sell its Securities to or through underwriters, dealers, placement agents or other intermediaries and the Corporation may also offer and sell its Securities directly to one or more purchasers or through agents in negotiated transactions, block trades, equity lines of credit or a combination of these methods, subject to obtaining any applicable exemption from registration requirements. The Securities offered pursuant to any Prospectus Supplement may be sold from time to time in one or more transactions at:
| - | a fixed price or prices, which may be changed from time to time; |
| - | market prices prevailing at the time of sale; |
| - | prices related to such prevailing market prices; or |
| - | other negotiated prices, including sales in transactions that are deemed to be “at-the-market” distribution” as defined in National Instrument 44-102 – Shelf Distributions, including sales made directly on the TSX, the NASDAQ or other existing trading markets for the Securities. |
The Corporation may only offer and sell the Securities pursuant to a Prospectus Supplement during the 25-month period that this Prospectus, including any amendments hereto, remains effective. The Prospectus Supplements for any of the Securities being offered thereby will set forth the terms of the offering of such Securities, including the type of Securities being offered, the name or names of any underwriters, dealers, placement agents, other intermediaries or agents, the purchase price of such Securities, the proceeds to the Corporation from such sale, any underwriting commissions or discounts and other items constituting compensation and any discounts or concessions allowed or re-allowed or paid to underwriters, dealers, placement agents, other intermediaries or agents. Only underwriters, dealers, placement agents, other intermediaries or agents so named in the Prospectus Supplements are deemed to be underwriters in connection with the Securities offered thereby.
| 7 |
In connection with the sale of Securities, underwriters, dealers, placement agents, other intermediaries or agents may receive compensation from the Corporation or from purchasers of Securities for whom they may act as intermediary or agents in the form of discounts, concessions or commissions. Underwriters, dealers, placement agents, other intermediaries or agents that participate in the distribution of Securities may be deemed to be underwriters and any discounts or commissions received by them from the Corporation and any profit on the resale of securities by them may be deemed to be underwriting discounts and commissions under applicable securities legislation.
If so indicated in the applicable Prospectus Supplements, the Corporation may authorize dealers, placement agents, other intermediaries or other persons acting as its agents to solicit offers by certain institutions to purchase the Securities directly from the Corporation pursuant to contracts providing for payment and delivery on a future date. These contracts will be subject only to the conditions set forth in the applicable Prospectus Supplements, which will also set forth the commission payable for solicitation of these contracts.
Any offering of Preferred Shares, Warrants, Units or Subscription Receipts will be a new issue of securities with no established trading market. Unless otherwise specified in the applicable Prospectus Supplements, the Preferred Shares, Warrants, Units or Subscription Receipts will not be listed on any securities or stock exchange or on any automated dealer quotation system. Unless otherwise specified in the applicable Prospectus Supplements, there is no market through which the Preferred Shares, Warrants, Units or Subscription Receipts may be sold and purchasers may not be able to resell Preferred Shares, Warrants, Units or Subscription Receipts purchased under this Prospectus or any Prospectus Supplement. This may affect the pricing of the Preferred Shares, Warrants, Units or Subscription Receipts in the secondary market, the transparency and availability of trading prices, the liquidity of the Securities, and the extent of issuer regulation. Certain dealers may make a market in the Preferred Shares, Warrants, Units or Subscription Receipts.
The Prospectus Supplements will set forth the terms of the offering of Securities, including:
| - | the name or the names of any underwriters, dealers, placement agents, other intermediaries or agents, if any; |
| - | the purchase price of, and form of consideration for, the Securities and the proceeds; |
| - | any delayed delivery arrangements; |
| - | any underwriting commissions, fees, discounts and other items constituting underwriters’ compensation; |
| - | the offering price; |
| - | any discounts or concessions allowed or re-allowed or paid to dealers; and |
| - | any other securities exchanges on which the Securities may be listed, if any. |
Only the underwriters, dealers, placement agents, other intermediaries or agents named in a Prospectus Supplement are deemed to be underwriters in connection with the Securities offered by that Prospectus Supplement.
The Common Shares may be sold, from time to time in one or more transactions at a fixed price or prices that may be changed or at market prices prevailing at the time of sale, at prices related to such prevailing market price or at negotiated prices.
Under agreements that may be entered into by IMV, underwriters, dealers, placement agents, other intermediaries or agents who participate in the distribution of Securities may be entitled to indemnification by the Corporation against certain liabilities, including liabilities under any applicable Canadian provincial securities legislation, or to contributions with respect to payments that such underwriters, dealers or agents may be required to make in that respect.
| 8 |
In connection with an offering, the underwriters, dealers, placement agents, other intermediaries or agents, if any, may over-allot or effect transactions that stabilize or maintain the market price of the Common Shares at a level above that which might otherwise prevail in the open market. Such transactions, if commenced, may be discontinued at any time and would be subject to applicable law.
By Underwriters, Dealers, Placement Agents or Other Intermediaries
If underwriters, dealers, placement agents or other intermediaries are used in the sale, the Securities will be acquired by such underwriters, dealers, placement agents or other intermediaries for their own account, as principals, and may be resold from time to time in one or more transactions, including negotiated transactions, at a fixed public offering price or at varying prices determined at the time of sale. Any public offering price and any discounts or concessions allowed or re-allowed or paid to underwriters, dealers, placement agents or other intermediaries may be changed from time to time. Unless otherwise set forth in the Prospectus Supplements relating thereto, the obligations of underwriters, dealers, placement agents or other intermediaries to purchase the Securities will be subject to certain conditions, but the underwriters, dealers, placement agents or other intermediaries will be obligated to purchase all of the Securities offered by the Prospectus Supplements if any of such Securities are purchased. The Corporation may agree to pay the underwriters, dealers, placement agents or other intermediaries a fee or commission for various services relating to the offering of any Securities. Any such fees or commissions will be paid out of the general corporate funds of the Corporation.
In compliance with the guidelines of the Financial Regulatory Authority Inc. (“FINRA”) and subject to the approval of FINRA, the maximum aggregate value of all compensation to be received by any FINRA member or independent broker-dealer will not exceed 8% of the gross proceeds from the sale of Securities pursuant to this Prospectus and any applicable Prospectus Supplement. If 5% or more of the net proceeds of any offering of Securities made under this Prospectus will be received by a FINRA member participating in the offering or affiliates or associated persons of such FINRA member, the offering will be conducted in accordance with FINRA Rule 5121 (or any successor rule).
By Agents
The Securities may also be sold through agents designated by the Corporation. Any agent involved will be named, and any fees or commissions payable by the Corporation to such agent will be set forth, in the applicable Prospectus Supplements. Any such fees or commissions will be paid out of the general corporate funds of the Corporation. Unless otherwise indicated in the Prospectus Supplements, any agent will be acting on a best efforts basis for the period of its appointment.
Direct Sales
Securities may also be sold directly by the Corporation at such prices and upon such terms as agreed to by the Corporation and the purchaser. In this case, no underwriters, dealers, placement agents, other intermediaries or agents would be involved in the offering.
General Information
Underwriters, dealers and agents that participate in the distribution of Securities may be deemed to be underwriters and any commissions received by them from the Corporation and any profit on the resale of Securities by them may be deemed to be underwriting commissions under the U.S. Securities Act of 1933, as amended.
Underwriters or agents who participate in the distribution of Securities may be entitled under agreements to be entered into with the Corporation to indemnification by the Corporation against certain liabilities, including liabilities under Canadian provincial and United States securities legislation, or to contribution with respect to payments which such underwriters or agents may be required to make in respect thereof. Such underwriters or agents may be customers of, engage in transactions with, or perform services for, us in the ordinary course of business.
| 9 |
The Corporation may enter into derivative transactions with third parties, or sell securities not covered by this Prospectus to third parties in privately negotiated transactions. If the applicable Prospectus Supplement indicates, in connection with those derivatives, the third parties may sell Securities covered by this Prospectus and the applicable Prospectus Supplement, including in short sale transactions. If so, the third parties may use Securities pledged by the Corporation or borrowed from the Corporation or others to settle those sales or to close out any related open borrowings of stock, and may use Securities received from the Corporation in settlement of those derivatives to close out any related open borrowings of stock. The third parties in such sale transactions will be identified in the applicable Prospectus Supplement.
One or more firms, referred to as “remarketing firms”, may also offer or sell the Securities, if the Prospectus Supplement so indicates, in connection with a remarketing arrangement upon their purchase. Remarketing firms will act as principals for their own accounts or as agents for the Corporation. These remarketing firms will offer or sell the Securities in accordance with the terms of the Securities. The Prospectus Supplement will identify any remarketing firm and the terms of its agreement, if any, with the Corporation and will describe the remarketing firm’s compensation. Remarketing firms may be deemed to be underwriters in connection with the Securities they remarket.
In connection with any offering of Securities, other than an “at-the-market” distribution, underwriters may over-allot or effect transactions which stabilize or maintain the market price of the Securities offered at a level above that which might otherwise prevail in the open market. Such transactions may be commenced, interrupted or discontinued at any time. With respect to an “at-the-market” distribution, no underwriter or dealer involved in the distribution, no affiliate of such an underwriter or dealer and no person or company acting jointly or in concert with such an underwriter or dealer will over-allot Securities in connection with the distribution or effect any other transactions that are intended to stabilize or maintain the market price of the Securities.
IMV’s authorized share capital consists of an unlimited number of Common Shares and Preferred Shares issuable in series, all without par value. As of June 4, 2018, a total of 43,221,570 Common Shares and no Preferred Shares are issued and outstanding.
On May 2, 2018, the Corporation filed articles of amendment to give effect to a consolidation of its Common Shares on the basis of 1 post-consolidation Common Share for each 3.2 pre-consolidation Common Shares.
Common Shares
The Common Shares of the Corporation rank junior to the Preferred Shares with respect to the payment of dividends, return of capital and distribution of assets in the event of liquidation, dissolution or winding-up of the Corporation. Subject to the prior rights of the holders of Preferred Shares, the holders of Common Shares are entitled to receive dividends as and when declared by the Board of Directors of the Corporation. In the event of liquidation, dissolution or winding-up of the Corporation, subject to the prior rights of the holders of Preferred Shares, the holders of Common Shares are entitled to receive all the remaining property and assets of the Corporation. The holders of Common Shares are entitled to receive notice of and to attend and to vote at all meetings of the shareholders of the Corporation and each Common Share, when represented at any meeting of the shareholders of the Corporation, carries the right to one vote.
Preferred Shares
The Preferred Shares of the Corporation are issuable from time to time in one or more series as determined by the Board of Directors of the Corporation. The Board of Directors of the Corporation may determine, before issuance, the designation, rights, privileges and restrictions attached to each series of Preferred Shares including the rate of preferential dividends, the dates of payment thereof, the redemption price and the terms of redemption, voting rights and conversion rights (if any), the whole subject to the filing of articles of amendment setting forth the designation, rights, privileges, restrictions, conditions and limitations attaching to the Preferred Shares of such series and the issuance of a certificate of amendment in respect thereof. If any cumulative dividends or amounts payable on return of capital in respect of a series of Preferred Shares are not paid in full, the Preferred Shares of all series shall participate rateably in respect of accumulated dividends and return of capital. The holders of Preferred Shares are entitled to priority over holders of any Common Shares of the Corporation with respect to the payment of dividends or the distribution of assets in the event of liquidation, dissolution or winding-up of the Corporation. Except as required by law or in accordance with any voting rights which may from time to time be attached to any series of Preferred Shares, the holders of the Preferred Shares as a class shall not be entitled to receive notice of, to attend or to vote at any meetings of the shareholders of the Corporation.
| 10 |
DESCRIPTION OF SUBSCRIPTION RECEIPTS
The following description of the terms of Subscription Receipts sets forth certain general terms and provisions of Subscription Receipts in respect of which a Prospectus Supplement may be filed. The particular terms and provisions of Subscription Receipts offered by any Prospectus Supplement, and the extent to which the general terms and provisions described below may apply thereto, will be described in the Prospectus Supplement filed in respect of such Subscription Receipts.
Subscription Receipts may be offered separately or in combination with one or more other Securities. The Subscription Receipts will be issued under a subscription receipt agreement. A copy of the subscription receipt agreement will be filed by the Corporation with the applicable securities commission or similar regulatory authorities after it has been entered into by IMV and will be available electronically at www.sedar.com. Pursuant to the subscription receipt agreement, original purchasers of Subscription Receipts will have a contractual right of rescission against the Corporation, following the issuance of the underlying Common Shares or other securities to such purchasers upon the surrender or deemed surrender of the Subscription Receipts, to receive the amount paid for the Subscription Receipts in the event that this Prospectus and any amendment thereto contains a misrepresentation or is not delivered to such purchaser, provided such remedy for rescission is exercised within 180 days from the closing date of the offering of Subscription Receipts.
The description of general terms and provisions of Subscription Receipts described in any Prospectus Supplement will include, where applicable:
| - | the number of Subscription Receipts offered; |
| - | the price at which the Subscription Receipts will be offered; |
| - | if other than Canadian dollars, the currency or currency unit in which the Subscription Receipts are denominated; |
| - | the procedures for the exchange of the Subscription Receipts into Common Shares or other securities; |
| - | the number of Common Shares or other securities that may be obtained upon exchange of each Subscription Receipt; |
| - | the designation and terms of any other Securities with which the Subscription Receipts will be offered, if any, and the number of Subscription Receipts that will be offered with each Security; |
| - | the terms applicable to the gross proceeds from the sale of the Subscription Receipts plus any interest earned thereon; |
| - | the material tax consequences of owning the Subscription Receipts; and |
| - | any other material terms, conditions and rights (or limitations on such rights) of the Subscription Receipts. |
The Corporation reserves the right to set forth in a Prospectus Supplement specific terms of the Subscription Receipts that are not within the options and parameters set forth in this Prospectus. In addition, to the extent that any particular terms of the Subscription Receipts described in a Prospectus Supplement differ from any of the terms described in this Prospectus, the description of such terms set forth in this Prospectus shall be deemed to have been superseded by the description of such differing terms set forth in such Prospectus Supplement with respect to such Subscription Receipts.
| 11 |
The following description, together with the additional information the Corporation may include in any applicable Prospectus Supplement, summarizes the material terms and provisions of the Warrants that the Corporation may offer under this Prospectus in one or more series. While the terms the Corporation has summarized below will apply generally to any Warrants that it may offer under this Prospectus, the Corporation will describe the particular terms of any series of Warrants that it may offer in more detail in the applicable Prospectus Supplement.
Unless the applicable Prospectus Supplement otherwise indicates, Warrants will be issued under and governed by the terms of one or more warrant indentures (each a “Warrant Indenture”) between the Corporation and a warrant trustee that the Corporation will name in the relevant Prospectus Supplements. Each warrant trustee will be a financial institution organized under the laws of Canada or any province thereof and authorized to carry on business as a trustee.
This summary of some of the provisions of the Warrants is not complete. The statements made in this Prospectus relating to any Warrant Indenture and Warrants to be issued under this Prospectus are summaries of certain anticipated provisions thereof and do not purport to be complete and are subject to, and are qualified in their entirety by reference to, all provisions of the applicable Warrant Indenture or Prospectus Supplement. Prospective purchasers should refer to the Prospectus Supplement and Warrant Indenture, if applicable, relating to the specific Warrants being offered for the complete terms of the Warrants. A copy of any Warrant Indenture relating to an offering of Warrants will be filed by the Corporation with the applicable securities regulatory authorities in Canada after the Corporation has entered into it and will be available electronically at www.sedar.com.
The applicable Prospectus Supplements relating to any Warrants offered by the Corporation will describe the particular terms of those Warrants and include specific terms relating to the offering. This description will include, where applicable:
| - | the designation and aggregate number of Warrants; |
| - | the price at which the Warrants will be offered; |
| - | the date on which the right to exercise the Warrants will commence and the date on which the right will expire; |
| - | the number of securities that may be purchased upon exercise of each Warrant and the price at which and currency or currencies in which the securities may be purchased upon exercise of each Warrant; |
| - | the designation and terms of any Securities with which the Warrants will be offered, if any, and the number of Warrants that will be offered with each Security; |
| - | the date or dates, if any, on or after which the Warrants and the other Securities with which the Warrants will be offered will be transferable separately; |
| - | whether the Warrants will be subject to redemption and, if so, the terms of such redemption provisions; |
| - | whether the Corporation will issue the Warrants as global securities and, if so, the identity of the depositary of the global securities; |
| - | whether the Warrants will be listed on any exchange; |
| - | material United States and Canadian federal income tax consequences of owning the Warrants; and |
| - | any other material terms or conditions of the Warrants. |
The Corporation reserves the right to set forth in a Prospectus Supplement specific terms of the Warrants that are not within the options and parameters set forth in this Prospectus. In addition, to the extent that any particular terms of the Warrants described in a Prospectus Supplement differ from any of the terms described in this Prospectus, the description of such terms set forth in this Prospectus shall be deemed to have been superseded by the description of such differing terms set forth in such Prospectus Supplement with respect to such Warrants.
| 12 |
The Corporation may issue Units comprised of one or more of the other Securities described in this Prospectus in any combination. Each Unit will be issued so that the holder of the Unit is also the holder of each Security included in the Unit. Thus, the holder of a Unit will have the rights and obligations of a holder of each included Security. The unit agreement, if any, under which a Unit is issued may provide that the Securities included in the Unit may not be held or transferred separately, at any time or at any time before a specified date.
The particular terms and provisions of Units offered by any Prospectus Supplement, and the extent to which the general terms and provisions described above may apply thereto, will be described in the Prospectus Supplement filed in respect of such Units.
The following table sets out the details of the issuance by the Corporation of Common Shares, options to purchase Common Shares, warrants to purchase Common Shares, deferred share units, if any, during the 12-month period before the date of this Prospectus:
| Security | Number(1) | Price(1) | Issuance Date | |||||||
| Common Shares(2) | 2,403,846 | $ | 4.16 | June 21, 2017 | ||||||
| Compensation options(3) | 144,231 | $ | 4.22 | June 21, 2017 | ||||||
| Common Shares(4) | 408 | N/A | (5) | June 26, 2017 | ||||||
| Deferred share units(6) | 24,202 | $ | 3.68 | June 30, 2017 | ||||||
| Common Shares(7) | 18,438 | $ | 2.30 | July 10, 2017 | ||||||
| Common Shares(7) | 15,156 | $ | 2.30 | July 19, 2017 | ||||||
| Deferred share units(8) | 25,074 | $ | 3.552 | September 30, 2017 | ||||||
| Common Shares(9) | 73,736 | $ | 1.92 | September 30, 2017 | ||||||
| Common Shares(7) | 8,906 | $ | 2.30 | October 19, 2017 | ||||||
| Common Shares(4) | 3,953 | N/A | (10) | November 15, 2017 | ||||||
| Common Shares(9) | 31,250 | $ | 1.92 | November 24, 2017 | ||||||
| Common Shares(7) | 78,125 | $ | 2.30 | November 24, 2017 | ||||||
| Common Shares(4) | 1,022 | N/A | (11) | December 1, 2017 | ||||||
| Common Shares(9) | 104,986 | $ | 1.92 | December 5, 2017 | ||||||
| Common Shares(7) | 46,875 | $ | 2.30 | December 5, 2017 | ||||||
| Common Shares(7) | 46,875 | $ | 2.30 | December 6, 2017 | ||||||
| Common Shares(7) | 11,250 | $ | 2.30 | December 12, 2017 | ||||||
| Common Shares(4) | 6,323 | N/A | (12) | December 19, 2017 | ||||||
| Deferred share units(13) | 12,101 | $ | 2.30 | December 31, 2017 | ||||||
| Common Shares(4) | 54,852 | N/A | (14) | January 3, 2018 | ||||||
| Common Shares(4) | 9,656 | N/A | (15) | January 8, 2018 | ||||||
| Stock Options(16) | 116,063 | $ | 7.04 | January 16, 2018 | ||||||
| Common Shares(4) | 1,692 | N/A | (5) | January 17, 2018 | ||||||
| Common Shares(4) | 7,631 | N/A | (17) | January 19, 2018 | ||||||
| Stock options(18) | 78,125 | $ | 7.04 | January 22, 2018 | ||||||
| Common Shares(4) | 9,148 | N/A | (19) | January 22, 2018 | ||||||
| Common Shares(20) | 2,246,094 | $ | 6.40 | February 15, 2018 | ||||||
| Common Shares(4) | 190,871 | N/A | (21) | February 26, 2018 | ||||||
| Common Shares(4) | 6,019 | N/A | (22) | February 28, 2018 | ||||||
| Stock Options(31) | 390,625 | $ | 6.40 | March 21, 2018 | ||||||
| Common Shares(4) | 625 | $ | 3.20 | March 22, 2018 | ||||||
| Common Shares(4) | 3,025 | $ | 0.90 | March 22, 2018 | ||||||
| Common Shares(4) | 773 | N/A | (23) | March 26, 2018 | ||||||
| Common Shares(4) | 3,125 | $ | 3.20 | March 27, 2018 | ||||||
| Deferred share units(27) | 15,675 | $ | 6.24 | March 31, 2018 | ||||||
| Stock options(32) | 4,688 | $ | 6.40 | April 1, 2018 | ||||||
| Common Shares(4) | 5,084 | N/A | (24) | April 3, 2018 | ||||||
| 13 |
| Security | Number(1) | Price(1) | Issuance Date | |||||||
| Common Shares(4) | 1,702 | N/A | (25) | April 4, 2018 | ||||||
| Common Shares(4) | 1,972 | N/A | (26) | April 5, 2018 | ||||||
| Common Shares(4) | 10,144 | $ | 0.90 | April 11, 2018 | ||||||
| Common Shares(7) | 22,891 | $ | 2.30 | April 19, 2018 | ||||||
| Common Shares(7) | 17,469 | $ | 2.30 | April 23, 2018 | ||||||
| Common Shares(7) | 15,625 | $ | 2.30 | April 24, 2018 | ||||||
| Common Shares(4) | 1,721 | N/A | (28) | April 27, 2018 | ||||||
| Common Shares(4) | 10,938 | $ | 0.90 | April 30, 2018 | ||||||
| Common Shares(29) | 15,625 | $ | 2.53 | May 1, 2018 | ||||||
| Common Shares(7) | 2,344 | $ | 2.30 | May 1, 2018 | ||||||
| Common Shares(7) | 4,250 | $ | 2.30 | May 3, 2018 | ||||||
| Common Shares(30) | 12,839 | N/A | (30) | May 15, 2018 | ||||||
| Common Shares(7) | 62,500 | $ | 2.30 | May 17, 2018 | ||||||
| Common Shares(7) | 7,812 | $ | 2.30 | May 18, 2018 | ||||||
| Common Shares(7) | 18,467 | $ | 2.30 | May 23, 2018 | ||||||
| Common Shares(4) | 15,625 | $ | 2.53 | May 23, 2018 | ||||||
| Common Shares(4) | 15,625 | $ | 2.11 | May 23, 2018 | ||||||
| Common Shares(4) | 3,125 | $ | 3.20 | May 23, 2018 | ||||||
| Common Shares(4) | 15,625 | $ | 2.37 | May 23, 2018 | ||||||
| Common Shares(4) | 15,625 | $ | 2.11 | May 23, 2018 | ||||||
| Common Shares(7) | 18,467 | $ | 2.30 | May 23, 2018 | ||||||
| Common Shares(7) | 10,812 | $ | 2.30 | May 24, 2018 | ||||||
| Common Shares(7) | 4,250 | $ | 2.30 | May 25, 2018 | ||||||
| Common Shares(7) | 4,109 | $ | 2.30 | May 28, 2018 | ||||||
| Common Shares(7) | 31,250 | $ | 2.30 | May 30, 2018 | ||||||
| Common Shares(7) | 16,328 | $ | 2.30 | May 31, 2018 | ||||||
| Common Shares(7) | 6,250 | $ | 2.30 | June 1, 2018 | ||||||
| (1) | On May 2, 2018, the Corporation filed articles of amendment to give effect to a consolidation of its Common Shares on the basis of 1 post-consolidation Common Share for each 3.2 pre-consolidation Common Shares. Historical information with regards to issuance of Common Shares, options to purchase Common Shares, warrants to purchase Common Shares and Deferred share units (“DSUs”) has been amended to reflect the 3.2 to 1 consolidation. Fractions have been rounded up or down to the nearest whole number and prices have been rounded up or down to the nearest cent. |
| (2) | Common Shares issued pursuant to a bought-deal public offering of Common Shares (the “June 2017 Public Offering”). |
| (3) | Compensation options exercisable at a price of $4.22 per Common Share until June 21, 2019 issued as consideration to the underwriters of the June 2017 Public Offering. |
| (4) | Common Shares issued upon exercise of stock options. |
| (5) | Cashless exercise of 3,125 options. |
| (6) | DSUs issued pursuant to the Corporation’s deferred share unit plan (the “DSU Plan”) at a deemed price of $3.68 per DSU. Each DSU entitles the holder thereof to receive one Common Share on the terms and conditions set forth in the DSU Plan. |
| (7) | Common Shares issued upon exercise of common share purchase warrants (the “2016 Warrants”) being part of units issued pursuant to a bought-deal private placement of units (the “June 2016 Private Placement”). Each 2016 Warrant entitles its holder to purchase one Common Share at a price of $2.30 per Common Share until June 8, 2018. |
| (8) | DSUs issued pursuant to the DSU Plan at a deemed price of $3.55 per DSU. Each DSU entitles the holder thereof to receive one Common Share on the terms and conditions set forth in the DSU Plan. |
| (9) | Common Shares issued upon exercise of non-transferable compensation options exercisable at a price of $1.92 per Common Share until June 8, 2018 issued as a consideration to the underwriters of the June 2016 Private Placement. |
| (10) | Cashless exercise of 7,813 options. |
| (11) | Cashless exercise of 1,885 options. |
| (12) | Cashless exercise of 9,375 options. |
| (13) | DSUs issued pursuant to the DSU Plan at a deemed price of $7.36 per DSU. Each DSU entitles the holder thereof to receive one Common Share on the terms and conditions set forth in the DSU Plan. |
| (14) | Cashless exercise of 78,188 options. |
| (15) | Cashless exercise of 10,938 options. |
| (16) | Grant of stock options governed by the Corporation’s stock option plan exercisable at a price of $7.04 per Common Share until January 16, 2023. |
| (17) | Cashless exercise of 8,738 options. |
| (18) | Grant of stock options governed by the Corporation’s stock option plan exercisable at a price of $7.04 per Common Share until January 22, 2023. |
| (19) | Cashless exercise of 13,438 options. |
| 14 |
| (20) | Common Shares issued pursuant to the February 2018 Public Offering. |
| (21) | Cashless exercise of 293,438 options. |
| (22) | Cashless exercise of 9,750 options. |
| (23) | Cashless exercise of 1,563 options. |
| (24) | Cashless exercise of 7,813 options. |
| (25) | Cashless exercise of 1,992 options. |
| (26) | Cashless exercise of 2,306 options. |
| (27) | DSUs issued pursuant to the DSU Plan at a deemed price of $6.24 per DSU. Each DSU entitles the holder thereof to receive one Common Share on the terms and conditions set forth in the DSU Plan. |
| (28) | Cashless exercise of 2,020 options. |
| (29) | Compensation options exercisable at a price of $2.528 per Common Share until December 9, 2018 issued as consideration to the underwriters of the December 2016 Private Placement. |
| (30) | Common shares issued upon redemption of 26,051 DSUs in accordance with the terms of the DSU Plan. |
| (31) | Grant of stock options governed by the Corporation’s stock option plan exercisable at a price of $6.40 per Common Share until March 21, 2023. |
| (32) | Grant of stock options governed by the Corporation’s stock option plan exercisable at a price of $6.40 per Common Share until April 1, 2023. |
The Common Shares are currently listed on the TSX under the symbol “IMV” and NASDAQ under the symbol “IMV”.
The following table provides the price ranges and trading volume of the Common Shares on the TSX for the periods indicated below:
Price Ranges(1) | Total Cumulative Volume(1) | |||||||||||
| High ($) | Low ($) | |||||||||||
| June 2017 | $ | 4.26 | $ | 3.58 | 429,493 | |||||||
| July 2017 | $ | 4.48 | $ | 3.46 | 621,775 | |||||||
| August 2017 | $ | 3.94 | $ | 3.55 | 370,905 | |||||||
| September 2017 | $ | 3.87 | $ | 3.36 | 528,076 | |||||||
| October 2017 | $ | 4.96 | $ | 3.33 | 1,342,138 | |||||||
| November 2017 | $ | 5.38 | $ | 4.32 | 1,008,102 | |||||||
| December 2017 | $ | 8.16 | $ | 5.06 | 1,514,423 | |||||||
| January 2018 | $ | 8.03 | $ | 5.98 | 1,103,615 | |||||||
| February 2018 | $ | 6.98 | $ | 5.82 | 589,178 | |||||||
| March 2018 | $ | 6.66 | $ | 5.92 | 361,870 | |||||||
| April 2018 | $ | 7.10 | $ | 5.18 | 551,825 | |||||||
| May 2018 | $ | 9.25 | $ | 6.11 | 1,439,544 | |||||||
| June 1-4, 2018 | $ | 9.49 | $ | 8.06 | 199,580 | |||||||
| (1) | On May 2, 2018, the Corporation filed articles of amendment to give effect to a consolidation of its Common Shares on the basis of 1 post-consolidation Common Share for each 3.2 pre-consolidation Common Shares. The post-consolidation Common Shares began trading on TSX on May 10, 2018. Historical trading prices and volumes have been amended to reflect the 3.2 for 1 consolidation. Fractions have been rounded up or down to the nearest whole number and prices have been rounded up or down to the nearest cent. |
| 15 |
An investment in the Corporation’s securities involves risk. Before you invest in the Securities, you should carefully consider the risks contained in or incorporated by reference into this Prospectus and any applicable Prospectus Supplement, including the risks described below and in the AIF and Annual MD&A, which are incorporated by reference into this Prospectus. The discussion of risks related to the business of the Corporation contained in or incorporated by reference into this Prospectus comprises material risks of which the Corporation is aware. If any of the events or developments described actually occurs, the business, financial condition or results of operations of the Corporation would likely be adversely affected.
Risks Relating to the Securities
The share price has been and is likely to continue to be volatile and an investment in Common Shares may suffer a decline in value.
You should consider an investment in Common Shares as risky and invest only if you can withstand a significant loss and wide fluctuations in the market value of your investment. The Corporation receives only limited attention by securities analysts and frequently experience an imbalance between supply and demand for Common Shares. The market price of the Common Shares has been highly volatile and is likely to continue to be volatile. This leads to a heightened risk of securities litigation pertaining to such volatility. Factors such as the financial position of the Corporation and the ability of the Corporation to continue as a going concern; the ability to raise additional capital; the progress of the clinical trials; the ability to obtain partners and collaborators to assist with the future development of the products; general market conditions; announcements of technological innovations or new product candidates by the Corporation, the Corporation collaborators or its competitors; published reports by securities analysts; developments in patent or other intellectual property rights; public concern as to the safety and efficacy of drugs that the Corporation and its competitors develop; and shareholder interest in the Common Shares all contribute to the volatility of the share price.
Future sales of Common Shares by the Corporation or by its existing shareholders could cause share price to fall.
The issuance of Common Shares by the Corporation could result in significant dilution in the equity interest of existing shareholders and adversely affect the market price of the Common Shares. Sales by existing shareholders of a large number of Common Shares in the public market and the issuance of Common Shares issued in connection with strategic alliances, or the perception that such additional sales could occur, could cause the market price of the Common Shares to decline and have an undesirable impact on the Corporation’s ability to raise capital.
Dilution of purchasers.
Purchasers who purchase Securities offered pursuant to this Prospectus may pay more for the Common Shares than the amounts paid by existing shareholders or security holders of the Corporation for their Common Shares. As a result, such purchasers may incur immediate and substantial dilution. Convertible securities have been issued and may be issued in the future by the Corporation at a lower price than the current market value of the Common Shares, consequently, purchasers who purchase Common Shares under the offering of Securities hereunder may incur substantial dilution in the near future.
No dividends have been paid on the Common Shares.
The Corporation has paid no cash dividends on any of its Common Shares to date and currently intends to retain its future earnings, if any, to fund the development growth of its businesses. In addition, the terms of any future debt or credit facility may preclude the Corporation from paying any dividends unless certain consents are obtained and certain conditions are met.
United States investors may not be able to obtain enforcement of civil liabilities against the Corporation.
The enforcement by investors of civil liabilities under the United States federal or state securities laws may be affected adversely by the fact that the Corporation is governed by the Canada Business Corporations Act, that the majority of the Corporation officers and directors are residents of Canada, and that all, or a substantial portion of their assets and a substantial portion of the Corporation assets, are located outside the United States. It may not be possible for investors to effect service of process within the United States on certain of its directors and officers or enforce judgments obtained in the United States courts against the Corporation or certain of the Corporation directors and officers based upon the civil liability provisions of United States federal securities laws or the securities laws of any state of the United States.
| 16 |
There is some doubt as to whether a judgment of a United States court based solely upon the civil liability provisions of United States federal or state securities laws would be enforceable in Canada against the Corporation or its directors and officers. There is also doubt as to whether an original action could be brought in Canada against the Corporation or its directors and officers to enforce liabilities based solely upon United States federal or state securities laws.
If the Corporation is characterized as a passive foreign investment company, U.S. holders may be subject to adverse U.S. federal income tax consequences.
U.S. investors should be aware that they could be subject to certain adverse U.S. federal income tax consequences in the event that the Corporation is classified as a “passive foreign investment company” (“PFIC”) for U.S. federal income tax purposes. The determination of whether the Corporation is a PFIC for a taxable year depends, in part, on the application of complex U.S. federal income tax rules, which are subject to differing interpretations, and the determination will depend on the composition of the Corporation’s income, expenses and assets from time to time and the nature of the activities performed by the Corporation’s officers and employees. The Corporation may be a PFIC in one or more prior tax years, in the current tax year and in subsequent tax years. Prospective investors should carefully read the tax discussion in any applicable Prospectus Supplement for more information and consult their own tax advisers regarding the likelihood and consequences of the Corporation being treated as a PFIC for U.S. federal income tax purposes, including the advisability of making certain elections that may mitigate certain possible adverse U.S. federal income tax consequences that may result in an inclusion in gross income without receipt of such income.
As a foreign private issuer, the Corporation is subject to different U.S. securities laws and rules than a domestic U.S. issuer, which may limit the information publicly available to its U.S. shareholders.
The Corporation is a foreign private issuer under applicable U.S. federal securities laws and, therefore, is not required to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act and related rules and regulations. As a result, the Corporation does not file the same reports that a U.S. domestic issuer would file with the SEC, although it will be required to file with or furnish to the SEC the continuous disclosure documents that the Corporation is required to file in Canada under Canadian securities laws. In addition, the Corporation’s officers, directors and principal shareholders are exempt from the reporting and “short swing” profit recovery provisions of Section 16 of the Exchange Act. Therefore, the Corporation’s shareholders may not know on as timely a basis when its officers, directors and principal shareholders purchase or sell securities of IMV as the reporting periods under the corresponding Canadian insider reporting requirements are longer. In addition, as a foreign private issuer, the Corporation is exempt from the proxy rules under the Exchange Act.
The Corporation may lose its foreign private issuer status in the future, which could result in significant additional costs and expenses to the Corporation.
In order to maintain its current status as a foreign private issuer, a majority of the Corporation’s Common Shares must be either directly or indirectly owned of record by non-residents of the United States unless the Corporation also satisfies one of the additional requirements necessary to preserve this status. The Corporation may in the future lose its foreign private issuer status if a majority of the Common Shares are owned of record in the United States and the Corporation fails to meet the additional requirements necessary to avoid loss of foreign private issuer status. The regulatory and compliance costs to the Corporation under U.S. federal securities laws as a U.S. domestic issuer may be significantly more than the costs the Corporation incurs as a Canadian foreign private issuer eligible to use MJDS. If the Corporation is not a foreign private issuer, it would not be eligible to use the MJDS or other foreign issuer forms and would be required to file periodic and current reports and registration statements on U.S. domestic issuer forms with the SEC, which are more detailed and extensive than the forms available to a foreign private issuer. In addition, the Corporation may lose the ability to rely upon exemptions from NASDAQ corporate governance requirements that are available to foreign private issuers.
| 17 |
CERTAIN CANADIAN FEDERAL INCOME TAX CONSiderations
The applicable Prospectus Supplement will describe certain Canadian federal income tax consequences to an investor who is a non-resident of Canada of acquiring any Securities offered thereunder, including whether the payments of distributions on the Securities will be subject to Canadian non-resident withholding tax.
CERTAIN U.S. FEDERAL INCOME TAX CONSIDERATIONS
The following is a general summary of certain material U.S. federal income tax considerations relevant to a U.S. Holder (as defined below) arising from and relating to the acquisition, ownership, and disposition of Common Shares acquired pursuant to this Prospectus.
This summary is for general information purposes only and does not purport to be a complete analysis or listing of all potential U.S. federal income tax consequences that may apply to a U.S. Holder arising from and relating to the acquisition, ownership, and disposition of Common Shares. In addition, this summary does not take into account the individual facts and circumstances of any particular U.S. Holder that may affect the U.S. federal income tax consequences to such U.S. Holder. Accordingly, this summary is not intended to be, and should not be construed as, legal or U.S. federal income tax advice with respect to any U.S. Holder. Except as discussed below, this summary does not discuss applicable income tax reporting requirements. This summary does not address the U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and non U.S. tax consequences to U.S. Holders of the acquisition, ownership, and disposition of Common Shares. Each prospective U.S. Holder should consult its own tax advisor regarding the U.S. federal, U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences relating to the acquisition, ownership, and disposition of Common Shares.
No legal opinion from U.S. legal counsel or ruling from the Internal Revenue Service (the “IRS”) has been requested, or will be obtained, regarding the U.S. federal income tax consequences of the acquisition, ownership, and disposition of Common Shares. This summary is not binding on the IRS, and the IRS is not precluded from taking a position that is different from, and contrary to, the positions taken in this summary. In addition, because the authorities upon which this summary is based are subject to various interpretations, the IRS and the U.S. courts could disagree with one or more of the positions taken in this summary.
Scope of this Summary
Authorities
This summary is based on the Internal Revenue Code of 1986, as amended (the “Code”), Treasury Regulations (whether final, temporary, or proposed), published rulings of the IRS, published administrative positions of the IRS, the Convention Between Canada and the United States of America with Respect to Taxes on Income and on Capital, signed September 26, 1980, as amended (the “Canada-U.S. Tax Convention”), and U.S. court decisions that are applicable and, in each case, as in effect and available, as of the date of this document. Any of the authorities on which this summary is based could be changed in a material and adverse manner at any time, and any such change could be applied on a retroactive or prospective basis. This summary does not discuss the potential effects, whether adverse or beneficial, of any proposed legislation that, if enacted, could be applied on a retroactive or prospective basis.
U.S. Holders
For purposes of this summary, the term “U.S. Holder” means a beneficial owner of Common Shares acquired pursuant to this Prospectus that is for U.S. federal income tax purposes:
| - | an individual who is a citizen or resident of the U.S.; |
| - | a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) organized under the laws of the U.S., any state thereof or the District of Columbia; |
| - | an estate whose income is subject to U.S. federal income taxation regardless of its source; or |
| 18 |
| - | a trust that (a) is subject to the primary supervision of a court within the U.S. and one or more U.S. persons has the authority to make all substantial decisions of the trust or (b) has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person. |
Non-U.S. Holders
For purposes of this summary, a “non-U.S. Holder” is a beneficial owner of Common Shares that is not a U.S. Holder and is not a partnership for U.S. federal income tax purposes. This summary does not address the U.S. federal income tax consequences to non-U.S. Holders arising from and relating to the acquisition, ownership, and disposition of Common Shares. Accordingly, a non-U.S. Holder should consult its own tax advisor regarding the U.S. federal, U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and foreign tax consequences (including the potential application of and operation of any income tax treaties) relating to the acquisition, ownership, and disposition of Common Shares.
U.S. Holders Subject to Special U.S. Federal Income Tax Rules Not Addressed
This summary does not address the U.S. federal income tax considerations applicable to U.S. Holders that are subject to special provisions under the Code, including, but not limited to, the following: (a) U.S. Holders that are tax-exempt organizations, qualified retirement plans, individual retirement accounts, or other tax-deferred accounts; (b) U.S. Holders that are financial institutions, underwriters, insurance companies, real estate investment trusts, or regulated investment companies; (c) U.S. Holders that are broker-dealers, dealers, or traders in securities or currencies that elect to apply a mark-to-market accounting method; (d) U.S. Holders that have a “functional currency” other than the U.S. dollar; (e) U.S. Holders that own Common Shares as part of a straddle, hedging transaction, conversion transaction, constructive sale, or other arrangement involving more than one position; (f) U.S. Holders that acquired Common Shares in connection with the exercise of employee stock options or otherwise as compensation for services; (g) U.S. Holders that hold Common Shares other than as a capital asset within the meaning of Section 1221 of the Code (generally, property held for investment purposes); or (h) U.S. Holders that own, have owned or will own (directly, indirectly, or by attribution) 10% or more of the total combined voting power of the outstanding Common Shares of the Corporation. This summary also does not address the U.S. federal income tax considerations applicable to U.S. Holders who are: (a) U.S. expatriates or former long-term residents of the U.S.; (b) persons that have been, are, or will be a resident or deemed to be a resident in Canada for purposes of the Tax Act; (c) persons that use or hold, will use or hold, or that are or will be deemed to use or hold Common Shares in connection with carrying on a business in Canada; (d) persons whose Common Shares constitute “taxable Canadian property” under the Tax Act; or (e) persons that have a permanent establishment in Canada for the purposes of the Canada-U.S. Tax Convention. U.S. Holders that are subject to special provisions under the Code, including, but not limited to, U.S. Holders described immediately above, should consult their own tax advisor regarding the U.S. federal, U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences relating to the acquisition, ownership and disposition of Common Shares.
If an entity or arrangement that is classified as a partnership for U.S. federal income tax purposes holds Common Shares, the U.S. federal income tax consequences to such entity and the partners (or other owners) of such partnership generally will depend on the activities of the partnership and the status of such partners. This summary does not address the tax consequences to any such owner. Partners of entities or arrangements that are classified as partnerships for U.S. federal income tax purposes should consult their own tax advisors regarding the U.S. federal income tax consequences arising from and relating to the acquisition, ownership, and disposition of Common Shares.
Ownership and Disposition of Common Shares
The following discussion is subject in its entirety to the rules described below under the heading “Passive Foreign Investment Company Rules”.
| 19 |
Taxation of Distributions
A U.S. Holder that receives a distribution, including a constructive distribution, with respect to a Common Share will be required to include the amount of such distribution in gross income as a dividend (without reduction for any Canadian or foreign income tax withheld from such distribution) to the extent of the current or accumulated “earnings and profits” of the Corporation, as computed for U.S. federal income tax purposes. To the extent that a distribution exceeds the current and accumulated “earnings and profits” of the Corporation, such distribution will be treated first as a tax-free return of capital to the extent of a U.S. Holder’s tax basis in the Common Shares and thereafter as gain from the sale or exchange of such Common Shares (see “Sale or Other Taxable Disposition of Common Shares”). However, the Corporation may not maintain the calculations of earnings and profits in accordance with U.S. federal income tax principles, and each U.S. Holder should therefore assume that any distribution by the Corporation with respect to the Common Shares will constitute ordinary dividend income. Dividends received on Common Shares generally will not be eligible for the “dividends received deduction” available to U.S. corporate shareholders receiving dividends from U.S. corporations.
Subject to applicable limitations and provided the Corporation is eligible for the benefits of the Canada – U.S. Tax Convention or the Common Shares are readily tradable on a United States securities market, dividends paid by the Corporation to non-corporate U.S. Holders, including individuals, generally will be eligible for the preferential tax rates applicable to long-term capital gains for dividends provided certain holding period and other conditions are satisfied, including that the Corporation not be classified as a PFIC in the tax year of distribution or in the preceding tax year. The dividend rules are complex, and each U.S. Holder should consult its own tax advisor regarding the application of such rules.
Sale or Other Taxable Disposition of Common Shares
A U.S. Holder will recognize gain or loss on the sale or other taxable disposition of Common Shares in an amount equal to the difference, if any, between (a) the amount of cash plus the fair market value of any property received and (b) such U.S. Holder’s tax basis in such Common Shares sold or otherwise disposed of. Any such gain or loss generally will be capital gain or loss, which will be long-term capital gain or loss if, at the time of the sale or other disposition, such Common Shares are held for more than one year.
Preferential tax rates apply to long-term capital gains of non-corporate U.S. Holders. There are currently no preferential tax rates for long-term capital gains of a U.S. Holder that is a corporation. Deductions for capital losses are subject to significant limitations under the Code. A U.S. Holder’s tax basis in Common Shares generally will be such U.S. Holder’s U.S. dollar cost for such Common Shares.
PFIC Status of the Corporation
If the Corporation is or becomes a PFIC, the preceding sections of this summary may not describe the U.S. federal income tax consequences to U.S. Holders of the ownership and disposition of Common Shares. The U.S. federal income tax consequences of owning and disposing of Common Shares if the Corporation is or becomes a PFIC are described below under the heading “Tax Consequences if the Corporation is a PFIC”.
A non-U.S. corporation is a PFIC for each tax year in which (i) 75% or more of its gross income is passive income (as defined for U.S. federal income tax purposes) (the “income test”) or (ii) on average for such tax year, 50% or more (by value) of its assets either produces or is held for the production of passive income (the “asset test”). For purposes of the PFIC provisions, “gross income” generally includes sales revenues less cost of goods sold, plus income from investments and from incidental or outside operations or sources, and “passive income” generally includes dividends, interest, certain rents and royalties, and certain gains from commodities or securities transactions. In determining whether or not it is a PFIC, a non-U.S. corporation is required to take into account its pro rata portion of the income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value).
Under certain attribution and indirect ownership rules, if the Corporation is a PFIC, U.S. Holders will generally be deemed to own their proportionate shares of the Corporation’s direct or indirect equity interests in any company that is also a PFIC (a “Subsidiary PFIC”), and will be subject to U.S. federal income tax on their proportionate share of (a) any “excess distributions”, as described below, on the stock of a Subsidiary PFIC and (b) a disposition or deemed disposition of the stock of a Subsidiary PFIC by the Corporation or another Subsidiary PFIC, both as if such U.S. Holders directly held the shares of such Subsidiary PFIC. In addition, U.S. Holders may be subject to U.S. federal income tax on any indirect gain realized on the stock of a Subsidiary PFIC on the sale or disposition of Common Shares. Accordingly, U.S. Holders should be aware that they could be subject to tax even if no distributions are received and no redemptions or other dispositions of the Corporation’s Common Shares are made.
| 20 |
The determination of PFIC status is inherently factual, is subject to a number of uncertainties, and can be determined only annually at the close of the tax year in question. Additionally, the analysis depends, in part, on the application of complex U.S. federal income tax rules, which are subject to differing interpretations. There can be no assurance that the Corporation will or will not be determined to be a PFIC for the current tax year or any prior or future tax year, and no opinion of legal counsel or ruling from the IRS concerning the status of the Corporation as a PFIC has been obtained or will be requested. U.S. Holders should consult their own U.S. tax advisors regarding the PFIC status of the Corporation.
Tax Consequences if the Corporation is a PFIC
If the Corporation is a PFIC for any tax year during which a U.S. Holder holds Common Shares, special rules may increase such U.S. Holder’s U.S. federal income tax liability with respect to the ownership and disposition of such shares. If the Corporation meets the income test or the asset test for any tax year during which a U.S. Holder owns Common Shares, the Corporation will be treated as a PFIC with respect to such U.S. Holder for that tax year and for all subsequent tax years, regardless of whether the Corporation meets the income test or the asset test for such subsequent tax years, unless the U.S. Holder elects to recognize any unrealized gain in the Common Shares or makes a timely and effective QEF Election or Mark-to-Market Election.
Under the default PFIC rules:
| - | any gain realized on the sale or other disposition (including dispositions and certain other events that would not otherwise be treated as taxable events) of Common Shares (including an indirect disposition of the stock of any Subsidiary PFIC) and any “excess distribution” (defined as a distribution to the extent it (together with all other distributions received in the relevant tax year) exceeds 125% of the average annual distributions received during the preceding three years) received on Common Shares or with respect to the stock of a Subsidiary PFIC will be allocated ratably to each day of such U.S. Holder’s holding period for the Common Shares; |
| - | the amount allocated to the current tax year and any year prior to the first year in which the Corporation was a PFIC will be taxed as ordinary income in the current year; the amount allocated to each of the other tax years (the “Prior PFIC Years”) will be subject to tax at the highest ordinary income tax rate in effect for the applicable class of taxpayer for that year; and |
| - | an interest charge will be imposed with respect to the resulting tax attributable to each Prior PFIC Year, which interest charge is not deductible by non-corporate U.S. Holders. |
A U.S. Holder that makes a timely and effective “mark-to-market” election under Section 1296 of the Code (a “Mark-to-Market Election”) or a timely and effective election to treat the Corporation and each Subsidiary PFIC as a “qualified electing fund” (a “QEF”) under Section 1295 of the Code (a “QEF Election”) may generally mitigate or avoid the PFIC consequences described above with respect to Common Shares.
U.S. Holders should be aware that, for each tax year, if any, that the Corporation is a PFIC, the Corporation can provide no assurances that it will satisfy the record keeping requirements of a PFIC, or that it will make available to U.S. Holders the information such U.S. Holders require to make a QEF Election with respect to the Corporation or any Subsidiary PFIC. U.S. Holders are urged to consult their own tax advisors regarding the potential application of the PFIC rules to the ownership and disposition of Common Shares, and the availability of certain U.S. tax elections under the PFIC rules.
A timely and effective QEF Election requires a U.S. Holder to include currently in gross income each year its pro rata share of the Corporation’s ordinary earnings and net capital gains, regardless of whether such earnings and gains are actually distributed. Thus, a U.S. Holder could have a tax liability with respect to such ordinary earnings or gains without a corresponding receipt of cash from the Corporation. If the Corporation is a QEF with respect to a U.S. Holder, the U.S. Holder’s basis in the Common Shares will be increased to reflect the amount of the taxed but undistributed income. Distributions of income that had previously been taxed will result in a corresponding reduction of basis in the Common Shares and will not be taxed again as a distribution to a U.S. Holder. Taxable gains on the disposition of Common Shares by a U.S. Holder that has made a timely and effective QEF Election are generally capital gains. A U.S. Holder must make a QEF Election for the Corporation and each Subsidiary PFIC if it wishes to have this treatment. To make a QEF Election, a U.S. Holder will need to have an annual information statement from the Corporation setting forth the ordinary earnings and net capital gains for the year. In general, a U.S. Holder must make a QEF Election on or before the due date for filing its income tax return for the first year to which the QEF Election will apply. Under applicable Treasury Regulations, a U.S. Holder will be permitted to make retroactive elections in particular circumstances, including if it had a reasonable belief that the Corporation was not a PFIC and filed a protective statement. If a U.S. Holder owns PFIC stock indirectly through another PFIC, separate QEF Elections must be made for the PFIC in which the U.S. Holder is a direct shareholder and the Subsidiary PFIC for the QEF rules to apply to both PFICs. Each U.S. Holder should consult its own tax advisor regarding the availability and desirability of, and procedure for, making a timely and effective QEF Election for the Corporation and any Subsidiary PFIC.
| 21 |
A Mark-to-Market Election may be made with respect to stock in a PFIC if such stock is “regularly traded” on a “qualified exchange or other market” (within the meaning of the Code and the applicable Treasury Regulations). A class of stock that is traded on one or more qualified exchanges or other markets is considered to be “regularly traded” for any calendar year during which such class of stock is traded in other than de minimis quantities on at least 15 days during each calendar quarter. If the Common Shares are considered to be “regularly traded” within this meaning, then a U.S. Holder generally will be eligible to make a Mark-to-Market Election with respect to its shares. However, there is no assurance that the Common Shares will be or remain “regularly traded” for this purpose. A Mark-to-Market Election may not be made with respect to the stock of any Subsidiary PFIC because such stock is not marketable. Hence, a Mark-to-Market Election will not be effective to eliminate the application of the default rules of Section 1291 of the Code, described above, with respect to deemed dispositions of Subsidiary PFIC stock or excess distributions with respect to a Subsidiary PFIC. A U.S. Holder that makes a timely and effective Mark-to-Market Election with respect to Common Shares generally will be required to recognize as ordinary income in each tax year in which the Corporation is a PFIC an amount equal to the excess, if any, of the fair market value of such shares as of the close of such taxable year over the U.S. Holder’s adjusted tax basis in such shares as of the close of such taxable year. A U.S. Holder’s adjusted tax basis in the Common Shares generally will be increased by the amount of ordinary income recognized with respect to such shares. If the U.S. Holder’s adjusted tax basis in the Common Shares as of the close of a tax year exceeds the fair market value of such shares as of the close of such taxable year, the U.S. Holder generally will recognize an ordinary loss, but only to the extent of net mark-to-market income recognized with respect to such shares for all prior taxable years. A U.S. Holder’s adjusted tax basis in its Common Shares generally will be decreased by the amount of ordinary loss recognized with respect to such shares. Any gain recognized upon a disposition of the Common Shares generally will be treated as ordinary income, and any loss recognized upon a disposition generally will be treated as an ordinary loss to the extent of net mark-to-market income recognized for all prior taxable years. Any loss recognized in excess thereof will be taxed as a capital loss. Capital losses are subject to significant limitations under the Code.
Each U.S. Holder should consult its own tax advisor regarding the availability and desirability of, and procedure for, making a timely and effective Mark-to-Market Election with respect to the Common Shares.
Additional Considerations
Additional Tax on Passive Income
Individuals, estates and certain trusts whose income exceeds certain thresholds will be required to pay a 3.8% Medicare surtax on “net investment income” including, among other things, dividends and net gain from disposition of property (other than property held in certain trades or businesses). Special rules apply to PFICs. U.S. Holders should consult with their own tax advisors regarding the effect, if any, of this tax on their ownership and disposition of Common Shares.
Receipt of Foreign Currency
The amount of any distribution paid in Canadian dollars to a U.S. Holder in connection with the ownership of the Common Shares, or on the sale, exchange or other taxable disposition of Common Shares, will be included in the gross income of a U.S. Holder as translated into U.S. dollars calculated by reference to the exchange rate prevailing on the date of actual or constructive receipt of the payment, regardless of whether the Canadian dollars are converted into U.S. dollars at that time. If the Canadian dollars received are not converted into U.S. dollars on the date of receipt, a U.S. Holder will have a basis in the Canadian dollars equal to their U.S. dollar value on the date of receipt. Any U.S. Holder who receives payment in Canadian dollars and engages in a subsequent conversion or other disposition of the Canadian dollars may have a foreign currency exchange gain or loss that would be treated as ordinary income or loss, and generally will be U.S. source income or loss for foreign tax credit purposes. Different rules apply to U.S. Holders who use the accrual method with respect to foreign currency. Each U.S. Holder should consult its own U.S. tax advisor regarding the U.S. federal income tax consequences of receiving, owning, and disposing of Canadian dollars.
| 22 |
Foreign Tax Credit
Subject to the PFIC rules discussed above, a U.S. Holder that pays (whether directly or through withholding) Canadian income tax with respect to dividends paid on the Common Shares generally will be entitled, at the election of such U.S. Holder, to receive either a deduction or a credit for such Canadian income tax paid. Generally, a credit will reduce a U.S. Holder’s U.S. federal income tax liability on a dollar-for-dollar basis, whereas a deduction will reduce a U.S. Holder’s income subject to U.S. federal income tax. This election is made on a year-by-year basis and applies to all foreign taxes paid (whether directly or through withholding) by a U.S. Holder during a year. Complex limitations apply to the foreign tax credit, including the general limitation that the credit cannot exceed the proportionate share of a U.S. Holder’s U.S. federal income tax liability that such U.S. Holder’s “foreign source” taxable income bears to such U.S. Holder’s worldwide taxable income. In applying this limitation, a U.S. Holder’s various items of income and deduction must be classified, under complex rules, as either “foreign source” or “U.S. source”. Generally, dividends paid by a foreign corporation should be treated as foreign source for this purpose, and gains recognized on the sale of stock of a foreign corporation by a U.S. Holder should be treated as U.S. source for this purpose, except as otherwise provided in an applicable income tax treaty, and if an election is properly made under the Code. However, the amount of a distribution with respect to the Common Shares that is treated as a “dividend” may be lower for U.S. federal income tax purposes than it is for Canadian federal income tax purposes, resulting in a reduced foreign tax credit allowance to a U.S. Holder. In addition, this limitation is calculated separately with respect to specific categories of income. The foreign tax credit rules are complex, and each U.S. Holder should consult its own U.S. tax advisor regarding the foreign tax credit rules.
Special rules apply to the amount of foreign tax credit that a U.S. Holder may claim on a distribution from a PFIC. Subject to such special rules, non-U.S. taxes paid with respect to any distribution in respect of stock in a PFIC are generally eligible for the foreign tax credit. The rules relating to distributions by a PFIC and their eligibility for the foreign tax credit are complex, and a U.S. Holder should consult its own tax advisor regarding their application to the U.S. Holder.
Backup Withholding and Information Reporting
Under U.S. federal income tax law and Treasury Regulations, certain categories of U.S. Holders must file information returns with respect to their investment in, or involvement in, a non-U.S. corporation. For example, U.S. return disclosure obligations (and related penalties) are imposed on U.S. Holders that hold certain specified foreign financial assets in excess of certain threshold amounts. The definition of “specified foreign financial assets” includes not only financial accounts maintained in foreign financial institutions, but also, if held for investment and not in an account maintained by certain financial institutions, any stock or security issued by a non-U.S. person, any financial instrument or contract held for investment that has an issuer or counterparty other than a U.S. person and any interest in a foreign entity. U.S. Holders may be subject to these reporting requirements unless their Common Shares are held in an account at certain financial institutions. Penalties for failure to file certain of these information returns are substantial. U.S. Holders should consult with their own tax advisors regarding the requirements of filing information returns on IRS Form 8938, and, if applicable, filing obligations relating to the PFIC rules, including possible reporting on IRS Form 8621.
Payments made within the U.S. or by a U.S. payor or U.S. middleman of (a) distributions on the Common Shares, and (b) proceeds arising from the sale or other taxable disposition of Common Shares generally will be subject to information reporting. In addition, backup withholding, currently at a rate of 24% for the 2018 to 2025 tax years (increasing to 28% for tax years after 2025), may apply to such payments if a U.S. Holder (a) fails to furnish such U.S. Holder’s correct U.S. taxpayer identification number (generally on IRS Form W-9), (b) furnishes an incorrect U.S. taxpayer identification number, (c) is notified by the IRS that such U.S. Holder has previously failed to properly report items subject to backup withholding, or (d) fails to certify, under penalty of perjury, that such U.S. Holder has furnished its correct U.S. taxpayer identification number and that the IRS has not notified such U.S. Holder that it is subject to backup withholding. Certain exempt persons generally are excluded from these information reporting and backup withholding rules. Backup withholding is not an additional tax. Any amounts withheld under the U.S. backup withholding rules will be allowed as a credit against a U.S. Holder’s U.S. federal income tax liability, if any, or will be refunded, if such U.S. Holder furnishes required information to the IRS in a timely manner. The information reporting and backup withholding rules may apply even if, under the Canada-U.S. Tax Convention, payments are exempt from the dividend withholding tax or otherwise eligible for a reduced withholding rate. This discussion of reporting requirements set forth above is not intended to constitute an exhaustive description of all reporting requirements that may apply to a U.S. Holder. A failure to satisfy certain reporting requirements may result in an extension of the time period during which the IRS can assess a tax, and under certain circumstances, such an extension may apply to assessments of amounts unrelated to any unsatisfied reporting requirements. Each U.S. Holder should consult its own tax advisor regarding the information reporting and backup withholding rules.
| 23 |
THE ABOVE SUMMARY IS NOT INTENDED TO CONSTITUTE A COMPLETE ANALYSIS OF ALL U.S. TAX CONSIDERATIONS APPLICABLE TO U.S. HOLDERS WITH RESPECT TO THE OWNERSHIP AND DISPOSITION OF COMMON SHARES. U.S. HOLDERS SHOULD CONSULT THEIR OWN TAX ADVISORS AS TO THE TAX CONSIDERATIONS APPLICABLE TO THEM IN THEIR PARTICULAR CIRCUMSTANCES.
Unless specified in the applicable Prospectus Supplement, certain Canadian legal matters will be passed upon on behalf of the Corporation by McCarthy Tétrault LLP. Certain legal matters relating to United States law will be passed upon on behalf of the Corporation by Troutman Sanders LLP. As of the date hereof, the partners and associates of McCarthy Tétrault LLP, as a group, and the partners and associates of Troutman Sanders LLP, as a group, beneficially own, directly or indirectly, less than 1% of the outstanding Common Shares.
Any Securities offered pursuant to this Prospectus, including by way of at-the-market offerings, will be conducted in accordance with applicable securities legislation in Canada and the United States, and, if applicable, will be subject to regulatory approval or exemptive relief.
AUDITOR, TRANSFER AGENT AND REGISTRAR
The auditor of the Corporation is PricewaterhouseCoopers LLP, Chartered Professional Accountants, Halifax, Nova Scotia, Canada.
The transfer agent and registrar for the Common Shares is Computershare Investor Services Inc., at its principal offices located in Toronto, Ontario, Canada or Montréal, Québec, Canada.
Albert Scardino and Wayne Pisano, directors of the Corporation, both reside outside of Canada and have appointed IMV Inc., #53-1344 Summer Street, Suite 412, Halifax, Nova Scotia, Canada, BH3 0A8, as agent for service of process.
Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process.
STATUTORY RIGHTS OF WITHDRAWAL AND RESCISSION
Securities legislation in certain of the provinces of Canada provides purchasers with the right to withdraw from an agreement to purchase securities. This right may be exercised within two business days after receipt or deemed receipt of a short form prospectus and any amendment. In several of the provinces of Canada, the securities legislation further provides a purchaser with remedies for rescission or, in some jurisdictions, revisions of the price or damages if the short form prospectus and any amendment contains a misrepresentation or is not delivered to the purchaser, provided that the remedies for rescission, revision of the price or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province for the particulars of these rights or consult with a legal advisor.
| 24 |
In an offering of Preferred Shares, Subscription Receipts, Warrants and Units (collectively, “Convertible Securities”), investors are cautioned that the statutory right of action for damages for a misrepresentation contained in the Prospectus and the accompanying Prospectus Supplements is limited, in certain provincial securities legislation, to the price at which such security is offered to the public under the prospectus offering. This means that, under the securities legislation of certain provinces, if the purchaser pays additional amounts upon conversion, exchange or exercise of the security, those amounts may not be recoverable under the statutory right of action for damages that applies in those provinces. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province for the particulars of this right of action for damages or consult with a legal advisor. By virtue of their purchase of Convertible Securities, original purchasers will have a contractual right of rescission against the Corporation in respect of the conversion, exchange or exercise of such Convertible Securities. The contractual right of rescission will entitle such original purchasers to receive the amount paid upon conversion, exchange or exercise, upon surrender of the securities issued to such purchaser upon conversion of such Convertible Securities, in the event that this Prospectus, as supplemented by an applicable Prospectus Supplement relating to such Convertible Securities, as amended, contains a misrepresentation, provided that the right of rescission is exercised within 180 days of the date of the purchase of the Convertible Securities. This contractual right of rescission will be consistent with the statutory right of rescission described under section 137 of the Securities Act (Nova Scotia), and is in addition to any other right or remedy available to original purchasers under section 137 the Securities Act (Nova Scotia) or otherwise to law. The purchaser should refer to any applicable provisions of the securities legislation of the province in which the purchaser resides for the particulars of these rights, or consult with a legal advisor.
The Corporation is incorporated under, and governed by, the laws of Canada. Many of its officers and directors and experts named in this Prospectus are resident outside of the United States, and a majority of their assets, and the assets of IMV, are located outside the United States. As a result, it may be difficult for U.S. investors to effect service of process within the United States upon those directors, officers or experts who are not residents of the United States, or to realize in the United States upon judgments of courts of the United States predicated upon civil liability of such directors, officers or experts under U.S. federal securities laws.
IMV has filed with the SEC, concurrently with the filing of its U.S. Registration Statement on Form F-10 of which this Prospectus forms a part, an appointment of agent for service of process on Form F-X. Under the Form F-X, IMV appointed C T Corporation System as its agent for service of process in the United States in connection with any investigation or administrative proceeding conducted by the SEC, and any civil suit or action brought against or involving IMV in a U.S. court arising out of or related to or concerning the offering of Securities under the U.S. Registration Statement. However, it may not be possible for investors to enforce outside the United States judgments against IMV obtained in the United States in any such actions, including actions predicated upon the civil liability provisions of the United States federal and state securities laws.
| 25 |
CERTIFICATE OF THE CORPORATION
Dated: June 5, 2018
This short form base shelf prospectus, together with the documents incorporated by reference, constitutes full, true and plain disclosure of all material facts relating to the securities offered in this prospectus as required by the securities legislation of British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Québec, Nova Scotia and Newfoundland and Labrador.
| /s/ Frederic Ors | /s/ Pierre Labbé | ||||
| Frederic
Ors Chief Executive Officer |
Pierre
Labbé Chief Financial Officer | ||||
| On behalf of the Board of Directors | |||||
| /s/ James Hall | /s/ Shermaine Tilley | ||||
| James
Hall Director |
Shermaine
Tilley Director | ||||
| C-1 |
US$30,000,000
Common Shares

IMV INC.
Prospectus Supplement
Piper Sandler & Co.
March 18, 2020